Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Nuclear imaging is a generic term for several imaging techniques. They all make use of small amounts of radioactive tracers administered to gain information about organs and tissues and represent an additional pillar in the diagnosis of CA. Nuclear imaging is not about functional, but more about structural assessment. While scintigraphy is two-dimensional or planar, positron emission tomography (PET) and single-photon emission computed tomography (SPECT) are three-dimensional.
- cardiac scintigraphy
- PET
- cardiac amyloidosis
1. Introduction
Cardiac amyloidosis (CA) is a disease characterized by deposition of misfolded protein fragments—called amyloid fibrils—in the heart. The two most frequent types of amyloidosis with cardiac involvement are light-chain amyloidosis (AL) and transthyretin amyloidosis (ATTR) [1]. TTR amyloidosis incorporates two subtypes: ATTRv (variant or hereditary amyloidosis) stands for all hereditary forms, whereas ATTRwt (wild-type amyloidosis) is defined by sporadic mutations of the transthyretin gene [2]. Secondary amyloidosis is a rare type of amyloidosis with deposition of serum amyloid A. It generally appears along with inflammatory diseases such as rheumatoid arthritis [3].
Although AL and ATTR have similar effects on myocardial function and patients typically present with signs and symptoms of heart failure, they are different conditions with various therapeutic approaches. Thereby, early initiation of therapy is crucial for the prognosis [4]. This is particularly worthwhile given the recent significant developments of treatment options for both types of amyloidosis.
2. Scintigraphy and Single-Photon Emission Computed Tomography
Scintigraphy is recommended in patients with suspected CA due to findings from TTE and CMR. In addition, patients with confirmed ATTR neuropathy or a positive test for a TTR gene mutation are also advised to undergo scintigraphy. Otherwise, it should also be considered in case of unexplained left ventricular wall thickening, bilateral carpal tunnel syndrome, and for individuals with a family history of amyloidosis [5].
Today, three bone avid radiotracers are used to perform scintigraphy in patients with suspected CA [6]. All of them are based on the radioisotope 99mTechnetium (99mTc). 99mTc-pyrophosphate (PYP) is approved by the Food and Drug Administration (FDA) and mainly used in the United States (US). 99mTc-hydroxymethylene diphosphonate (HMDP) and 99mTc-3,3-diphosphono-1,2-propanodicarobxylicacid (DPD) are applied in Europe, but rarely or not utilized in the US. The radiotracers are injected intravenously. Whole-body planar images and chest or cardiac SPECT images are obtained two to three hours post-injection. The myocardial uptake of the tracer is then compared to the uptake of the rib cage (typically as a heart-to-bone and heart-to-contralateral ratio). It is graded from 0 to 3 according to the Perugini grading scale (0: no uptake, 1: uptake less than rib, 2: uptake equal to rib, 3: uptake greater than rib). Grades 2 and 3 are strongly suggestive for the presence of cardiac ATTR. Grade 0 is not suggestive, and Grade 1 is considered equivocal (Figure 1). Scintigraphy should always be accompanied by the performance of serum and urine immunofixation and serum-free light-chain studies. In September 2019, the American Society of Nuclear Cardiology (ASNC) and the European Association of Nuclear Medicine (EANM) published practice points regarding scintigraphy in CA.
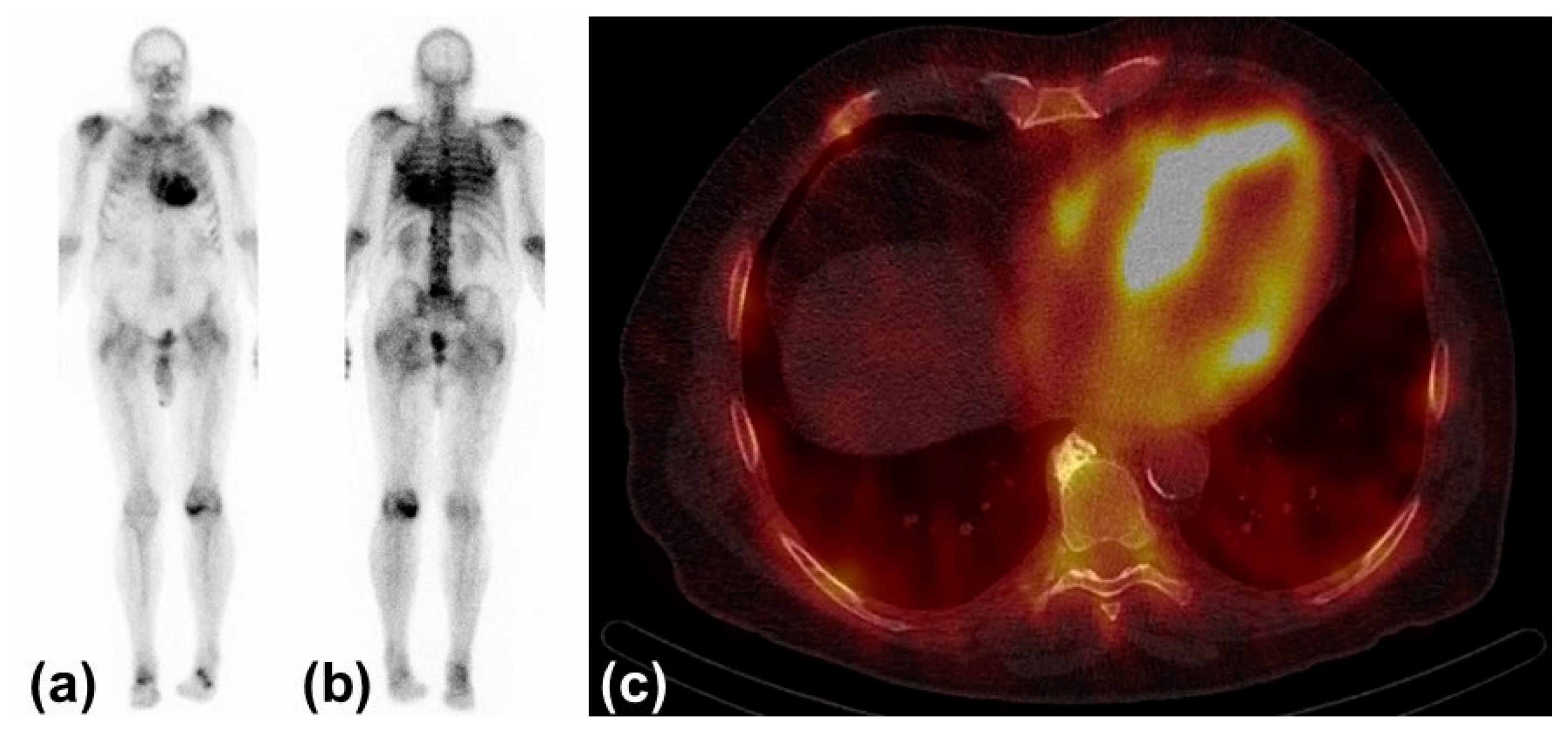
Figure 1. Typical findings in scintigraphy and single-photon emission computed tomography: (a) Anterior view of 99Tc-hydroxymethylene diphosphonate scintigraphy with myocardial uptake Perugini Grade 3. (b) Posterior view of the same study. (c) Left and right ventricular uptake of 99mTc-hydroxymethylene diphosphonate in single-photon emission computed tomography.
In the late twentieth century, knowledge about scintigraphy for the diagnosis of CA was not consolidated. A study from 1987 by Gertz et al. serves as an example by showing that only a loose association between scintigraphy and CA was established [7]. Further research revealed, both in amyloidosis and scintigraphy, that microcalcifications are responsible for the selectivity of bone avid radiotracers in CA. The density of microcalcifications is higher in ATTR compared to AL [8].
A large study published in 2016 highlighted the performance of scintigraphy for the diagnosis of cardiac ATTR. The combined finding of Grade 2 or 3 radiotracer uptake in the absence of monoclonal protein in serum and urine is associated with a specificity and a positive predictive value for ATTR CA of 100%. Radiotracer uptake alone had a sensitivity of >99% and a specificity of 86% for ATTR CA. False positive cases almost exclusively occurred in patients with AL CA [9]. Especially in patients with advanced amyloid deposition, scintigraphy using bone avid radiotracers is the best available tool for the discrimination of AL and ATTR CA [10]. Scintigraphy therefore plays an important role in the guidelines for the diagnosis of CA of both the American Heart Association and the European Society of Cardiology [11][12].
In addition, radiotracer uptake correlates positively with all-cause mortality and negatively with survival free of cardiac adverse events [5]. Another approach, which is used to evaluate the forecast, is the assess autonomic dysfunction as a non-specific finding in patients with CA resulting in arrhythmias. Here, 123I-metaiodobenzylguanidine (MIBG) is used as a radiotracer, analogous to norepinephrine, to derive the cardiac-to-mediastinum ratio. In combination with the heart rate response to atropine, it can be used as a predictor of prognosis in patients with ATTR CA [10][13].
3. Positron Emission Tomography
11C-Pittsburgh Compound B, 18F-Florbetapir, and 18F-Florbetaben are amyloid-binding radiotracers. They were not designed for cardiac imaging but, i.e., for imaging of beta-amyloid deposits in the brain in Alzheimer’s disease. PET is a functional nuclear imaging technique using computed tomography (CT) or magnetic resonance tomography (MRT), which is therefore not only eligible for CA but also systemic amyloidosis. Amyloid-binding radiotracers enable a quantification of the global amyloid burden but also of a regional burden, such as the myocardium. The parameters measured are the self-explanatory target-to-background ratio, myocardial standard uptake value (SUV, parameter to measure radioactivity of the studied tissue), and myocardial retention index (derived from SUV in different phases after radiotracer injection). The direct binding of the tracers to the agent causing the disease allows early detection of the disease, even before structural changes appear. Early detection is crucial for effective treatment. Distinction between the different types of CA is not feasible with this technique. Due to possible quantification of deposits, PET has the potential for serial imaging in order to monitor response to treatment [14].
Singh and Dorbala provide an overview of several studies conducted on PET in CA. The studies are limited by small sample sizes and a lack of standardized protocols for image acquisition. This can be illustrated with one specific publication commented on in the above work, which studied a new radiotracer (amyloid-binding 18F-Flutemetamol) in only 17 patients undergoing PET (with CT or MRT). Images were acquired more than one hour after injection of the radiotracer, which is rather late compared to other amyloid-binding radiotracers. Only a small percentage of patients with CA showed relevant radiotracer uptake [15][16][17].
However, further research with larger sample sizes and standardized protocols is needed before PET is applied in clinical routine.
This entry is adapted from the peer-reviewed paper 10.3390/biomedicines10040903
References
- Benson, M.D.; Buxbaum, J.N.; Eisenberg, D.S.; Merlini, G.; Saraiva, M.J.M.; Sekijima, Y.; Sipe, J.D.; Westermark, P. Amyloid nomenclature 2020: Update and recommendations by the International Society of Amyloidosis (ISA) nomenclature committee. Amyloid 2020, 27, 217–222.
- Gertz, M.A.; Benson, M.D.; Dyck, P.J.; Grogan, M.; Coelho, T.; Cruz, M.; Berk, J.L.; Plante-Bordeneuve, V.; Schmidt, H.; Merlini, G. Diagnosis, Prognosis and Therapy of Transthyretin Amyloidosis. J. Am. Coll. Cardiol. 2015, 66, 2451–2466.
- Ash, S.; Shorer, E.; Bs, D.R.; Vo, M.; Bs, J.G.; Golamari, R.; Jain, R.; Jain, R. Cardiac amyloidosis—A review of current literature for the practicing physician. Clin. Cardiol. 2021, 44, 322–331.
- Sperry, B.W.; Vranian, M.N.; Hachamovitch, R.; Joshi, H.; Ikram, A.; Phelan, D.; Hanna, M. Subtype-Specific Interactions and Prognosis in Cardiac Amyloidosis. J. Am. Heart Assoc. 2016, 5, e002877.
- Dorbala, S.; Ando, Y.; Bokhari, S.; Dispenzieri, A.; Falk, R.H.; Ferrari, V.A.; Fontana, M.; Gheysens, O.; Gillmore, J.D.; Glaudemans, A.W.J.M.; et al. ASNC/AHA/ASE/EANM/HFSA/ISA/SCMR/SNMMI Expert Consensus Recommendations for Multimodality Imaging in Cardiac Amyloidosis: Part 1 of 2—Evidence Base and Standardized Methods of Imaging. Circ. Cardiovasc. Imaging 2021, 14, e000029.
- Hotta, M.; Minamimoto, R.; Awaya, T.; Hiroe, M.; Okazaki, O.; Hiroi, Y. Radionuclide Imaging of Cardiac Amyloidosis and Sarcoidosis: Roles and Characteristics of Various Tracers. RadioGraphics 2020, 40, 2029–2041.
- Gertz, M.A.; Brown, M.L.; Hauser, M.F.; Kyle, R.A. Utility of Technetium Tc 99m Pyrophosphate Bone Scanning in Cardiac Amyloidosis. Arch. Intern. Med. 1987, 147, 1039–1044.
- Stats, M.A.; Stone, J.R. Varying levels of small microcalcifications and macrophages in ATTR and AL cardiac amyloidosis: Implications for utilizing nuclear medicine studies to subtype amyloidosis. Cardiovasc. Pathol. 2016, 25, 413–417.
- Gillmore, J.D.; Maurer, M.S.; Falk, R.H.; Merlini, G.; Damy, T.; Dispenzieri, A.; Wechalekar, A.D.; Berk, J.L.; Quarta, C.C.; Grogan, M.; et al. Nonbiopsy Diagnosis of Cardiac Transthyretin Amyloidosis. Circulation 2016, 133, 2404–2412.
- Bokhari, S.; Shahzad, R.; Castaño, A.; Maurer, M.S. Nuclear imaging modalities for cardiac amyloidosis. J. Nucl. Cardiol. 2014, 21, 175–184.
- Garcia-Pavia, P.; Rapezzi, C.; Adler, Y.; Arad, M.; Basso, C.; Brucato, A.; Burazor, I.; Caforio, A.L.P.; Damy, T.; Eriksson, U.; et al. Diagnosis and treatment of cardiac amyloidosis: A position statement of the ESC Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2021, 42, 1554–1568.
- Kittleson, M.M.; Maurer, M.S.; Ambardekar, A.V.; Bullock-Palmer, R.P.; Chang, P.P.; Eisen, H.J.; Nair, A.P.; Nativi-Nicolau, J.; Ruberg, F.L.; On behalf of the American Heart Association Heart Failure and Transplantation Committee of the Council on Clinical Cardiology. Cardiac Amyloidosis: Evolving Diagnosis and Management: A Scientific Statement From the American Heart Association. Circulation 2020, 142, e7–e22.
- Algalarrondo, V.; Antonini, T.; Théaudin, M.; Chemla, D.; Benmalek, A.; Lacroix, C.; Castaing, D.; Cauquil, C.; Dinanian, S.; Eliahou, L.; et al. Cardiac Dysautonomia Predicts Long-Term Survival in Hereditary Transthyretin Amyloidosis After Liver Transplantation. JACC Cardiovasc. Imaging 2016, 9, 1432–1441.
- Dorbala, S.; Cuddy, S.; Falk, R.H. How to Image Cardiac Amyloidosis. JACC Cardiovasc. Imaging 2020, 13, 1368–1383.
- Singh, V.; Dorbala, S. Positron emission tomography for cardiac amyloidosis: Timing matters! J. Nucl. Cardiol. 2020, 29, 790–797.
- Papathanasiou, M.; Kessler, L.; Carpinteiro, A.; Hagenacker, T.; Nensa, F.; Umutlu, L.; Forsting, M.; Brainman, A.; Kleinschnitz, C.; Antoch, G.; et al. 18F-flutemetamol positron emission tomography in cardiac amyloidosis. J. Nucl. Cardiol. 2020, 29, 779–789.
- Sperry, B.W.; Bock, A.; DiFilippo, F.P.; Donnelly, J.P.; Hanna, M.; Jaber, W.A. Pilot Study of F18-Florbetapir in the Early Evaluation of Cardiac Amyloidosis. Front. Cardiovasc. Med. 2021, 8, 693194.
This entry is offline, you can click here to edit this entry!
