You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Infectious Diseases
Long COVID is a term coined by the World Health Organization (WHO) to describe a variety of persistent symptoms after acute SARS-CoV-2 infection. Long COVID has been demonstrated to affect various SARS-CoV-2-infected persons, independently of the acute disease severity.
- SARS-CoV-2
- COVID-19
- Long COVID
- Renin-Angiotensin System
1. The Central Nervous System
The persisting sequelae after SARS-CoV-2 infection involving the central nervous system (CNS) included predominant neurologic and psychiatric symptoms, such as memory and attention deficits, reduced capacity to do daily tasks, frequent headaches, changes in cutaneous sensation, autonomic dysfunction, chronic fatigue, and, in severe cases, delusions and paranoia [17,24,25]. Surprisingly, many patients showing these neurologic symptoms for more than a year after contracting COVID-19 are under 50 years old and were healthy and active before getting infected [26]. This supports the idea that the virus can have a serious impact on the CNS. In fact, imaging results of the UK Biobank cohort indicated specific areas of brain atrophy in COVID-19 patients, compared to a control group without COVID-19, highlighting a direct impact of the virus on the CNS [26]. Also, neuronal atrophy and degeneration of cranial nerves, including the olfactory nerve and the neighboring olfactory bulb, have previously been reported in patients with persistent hyposmia or anosmia after acute COVID-19 [27]. In agreement with these findings, patients with long COVID exhibited lower metabolic activity in their brain [26]. The etiology underlying these symptoms and alterations in the brain, however, remains unknown. Of note, affected zones, including the cerebral cortex and hippocampus, possess different members of the RAS system.
2. The Peripheral Nervous System
The peripheral nervous system (PNS) can be also affected by SARS-CoV-2 infection and show persistent symptoms for weeks or even months after the infection. Peripheral neuropathy symptoms differ depending on which nerves are damaged: motor, sensory, or autonomic. COVID-19 infection is thought to affect the autonomic nervous system (ANS). In fact, the well-known COVID-19 cytokine response storm is caused by sympathetic activity which causes pro-inflammatory cytokine release. Vagus nerve stimulation mediating anti-inflammatory responses highlight the relationship between SARS-CoV-2 infection and the PNS and suggest the ANS as a therapeutic target. Of note, vagus nerve dysfunction has also been reported in SARS-CoV-2 infection and proposed as a key pathophysiological hallmark of long COVID [28].
COVID-19-related autonomic dysfunction might be caused by the virus itself [29]. In fact, cohort studies have identified viral infections as a common precursor to autonomic diseases such as orthostatic hypotension and postural orthostatic tachycardia syndrome. These immune-mediated neurological disorders are caused by autoantibodies, such as those against α/β adrenoceptors and muscarinic receptors [30,31,32,33,34]. As a result, it could be possible that SARS-CoV-2 infection promotes autonomic dysfunctions through an autoimmune-mediated process. In agreement with this hypothesis, Guillain-Barré syndrome (GBS), a rare, autoimmune disorder that targets nerves, was associated with SARS-CoV-2 infection [35,36]. These effects are described in macrophage activation syndrome induced by RAS dysfunction, which is known to cause an alteration of the innate immunity responsible for autoimmune diseases.
Symptoms of fever, cough, interstitial pneumonia, hypo-ageusia, and hypo-anosmia were associated with GBS in conjunction with COVID-19 [37]. This hypothesis was also investigated by checking whether some symptoms of long COVID could be linked to a virus- or immune-mediated disturbance of the ANS, resulting in acute or long-term orthostatic intolerance syndromes. Orthostatic hypotension (OH), vasovagal syncope (VVS), and postural orthostatic tachycardia syndrome (POTS) are all orthostatic intolerance syndromes [38]. An aberrant autonomic reaction to orthostasis is central to the pathogenesis (standing up). In fact, blood accumulates in the pelvis and legs when a healthy individual stands, decreasing venous return. Baroreceptors in the heart and aorta perceive this and respond by raising sympathetic neuronal and adrenergic tone (mediated by norepinephrine and epinephrine, respectively). This causes tachycardia followed by splanchnic vascular bed vasoconstriction, which enhances venous return to the heart [38]. The release of adrenaline and norepinephrine in orthostatic intolerance induces the significant tachycardia, which manifests as palpitations, dyspnea, and chest pain, which are typical symptoms of long COVID [39]. The poor control of heart rate variability (HRV) in long COVID patients also supports the autonomic dysfunction in these patients. This dysregulation was linked to long COVID symptoms such as fatigue and hypoxia [40]. Signs of peripheral nerve and muscular system dysfunction were also reported after SARS-CoV-2 infection. The persistent symptoms of myalgia, weakness (or exercise intolerance), sensory dysfunctions (primarily positive symptoms, such as paraesthesia and neuropathic pain), and dysautonomia were all linked to long COVID [24].
3. The Outcomes of Neuroinflammation
An important role of neuroinflammation has also been linked to long COVID. In fact, abnormal humoral and cellular immune responses, systemic inflammatory markers such as IL-6, and autoantibodies targeting cellular receptors could all be involved in systemic and neurological long COVID sequelae [37]. The full scope of long COVID neurological problems has yet to be determined. Neuroinflammation and neuronal injury observed in acute COVID-19 raise the possibility that infection could speed up or induce the development of neurodegenerative illnesses like Alzheimer’s or Parkinson’s. There is currently no evidence on the neurodevelopmental trajectories of children who typically have modest COVID-19 and show low neurologic or neuropsychiatric symptoms during or after acute illness. Because of broad endothelial activation, which commonly involves the brain, those who suffer from the unusual multisystem inflammatory syndrome in children (MIS-C) may be at a higher risk for neurological complications [26].
4. SARS-CoV-2 Entry into the CNS and PNS
Hematogenous or transsynaptic pathways involving the ACE2 receptor, which is found on the surface of a variety of cells, including neurons, astrocytes, endothelial, and smooth muscle cells of cerebral blood vessels, and skeletal muscle cells, facilitate SARS-CoV-2 cell entry into the CNS and PNS [41]. In the CNS, ACE2 receptors are predominantly expressed in the olfactory bulb, amygdala, hippocampus, middle temporal gyrus, posterior cingulate cortex, and the brainstem; therefore, hyposmia, mood disorders, cognitive impairment, sleep disorders, and dysautonomia have been linked to the dysfunction of these ‘ACE2-rich’ brain areas [17,40,42,43]. SARS-CoV-2 could indeed infect brain cells such as neurons, astrocytes, and microglial cells (and possibly oligodendrocytes) by binding to their ACE2 receptors and, as a result, after entering the ear-nose-throat (ENT) sphere, attacking the olfactory bulb on the floor of the cranial box through the olfactory epithelium just below the nasal cavity level. The involvement of the brainstem and cerebellum is critical evidence suggesting that these brain regions may be involved in several neurological manifestations of long COVID which are similar to myalgic encephalomyelitis or chronic fatigue syndrome (ME/CFS) and POTS [27].
5. The Complexity of RAS in the CNS and Its Impairing in Long COVID
Besides the endocrine RAS regulating water and electrolyte balance, it was demonstrated that neurons have intracrine and local types of RAS [44,45]. RAS regulates brain homeostasis primarily by the action of four angiotensin receptor subtypes: AT1R, AT2R, MasR, and angiotensin II type-4 receptor (AT4R). AT1R induces vasoconstriction, proliferation, inflammation, and oxidative stress, whereas AT2R and MasR mitigate AT1R’s actions. AT1Rs are divided into two types: AT1A and AT1B. The AT1A receptor is mostly found in brain regions that contribute to blood pressure and electrolyte balance homeostasis, whereas the AT1B receptor is found in structures like the cerebral cortex and hippocampus that are involved in memory and higher brain functions. AT4R regulates the release of dopamine and acetylcholine, as well as memory and learning consolidation [11,46]. AT2R is abundantly expressed in the brain. It plays a role in brain damage healing (i.e., axonal regeneration, neurite development) as well as inflammation reduction and vasodilation. In other words, AT2R has a neuroprotective function by helping neuronal survival and protecting the brain from damages. In this context, the AT2R protects against the harmful effects of AT1R activation, which occurs during SARS-CoV-2 infection [47,48]. It has been shown that SARS-CoV-2 has a brain tropism, and the neurological dysfunctions reported could be due to the impairment of RAS in the CNS (Figure 2). It has been shown that SARS-CoV-2 can infect nerve cells (such as neurons and astrocytes that express the ACE2 receptor) in vitro and in vivo [11,45,49,50,51,52]. The fact that SARS-CoV-2 impairs RAS pathways suggests that many nervous sequelae of long COVID could be explained by ACE2 receptor loss and AngII/AT1R over-activation. The virus binds to ACE2, resulting in its downregulation and a shift in the dynamic balance between the two faces of the RAS: (1) ACE/Ang II/AT1R, which has proinflammatory features, and (2) ACE2/Ang-(1-7)/AT2R, which has anti-inflammatory properties. This imbalance due to the overactivation of the Ang II/AT1R axis in the brain leads to hypertension, neuroinflammation, increased oxidative stress, BBB disruption, and neurotoxicity [11,42,46].
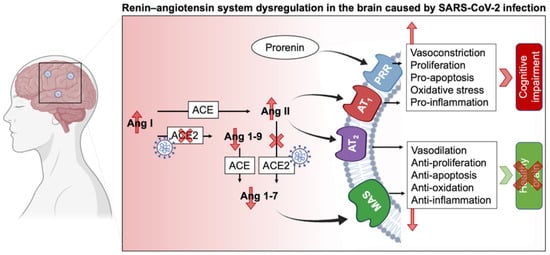
Figure 1. Schematic of RAS impairment in the brain duringSARS-CoV-2 infection. ACE: Angiotensin-converting enzyme; Ang: Angiotensin; AT1R: Angiotensin II receptor type 1; AT2R: Angiotensin II receptor type 2; MAS: Mas-related G protein-coupled receptors, PRR: Prorenin receptor.
The abundancy of RAS in the brain and its involvement in different physiological and cognitive processes explain the broad consequences of SARS-CoV-2 infection. The diversity of the RAS, notably via its two types, circulating and local RAS, emphasizes its important role in homeostasis. As such, circulating RAS affects nuclei in the hypothalamus and medulla via circumventricular organs, which are brain areas that lack a blood-brain barrier (BBB), and transmit to nuclei in the hypothalamus and medulla, while the independent local RAS of the brain, b-RAS, generates all components of the circulatory RAS [44]. On the other hand, the prorenin receptor (PRR) is widely expressed in neurons, and some microglial cells of many vascular brain regions as well as the brain cortex and basal ganglia [53]. Overstimulation of this system activates the Ang II/AT1R axis and thus may lead to cognitive impairment. Furthermore, PRR can establish its own signaling pathway and produce pro-oxidative effects. Although a link connecting PRR and neural development has been shown, the RAS-independent functions of PRR in the brain are yet unknown [17,54].
AT1R signaling can be also triggered by ACE overexpression [55] and results in (i) second messenger signaling including inositol trisphosphate, diacylglycerol, and arachidonic acid, as well as the (ii) activation of downstream effectors including phospholipases C, A, and D after G-protein coupling stimulation of AT1R by Ang II. The AT1R signaling cascade activates protein kinase C, Akt, intracellular protein kinases and serine/threonine kinases (such as mitogen-activated protein kinase (MAPK) family kinases). These facts highlight the diversity of the components that can be touched by RAS impairment and their consequent sequelae. For example, hypertrophy, vascular remodeling, and hyperplasia may arise from overactivation of the AT1R cascade [11,55,56]. The Ang II/AT2Rs counteract AT1R’s effects by promoting phospholipase A2 and activating multiple protein phosphatases as well as the nitric oxide (NO)/cyclic GMP pathway, triggering the release of arachidonic acid [25,48]. AT2R also suppresses cell growth and proliferation by blocking insulin and EGFR autophosphorylation. Furthermore, by inhibiting negative feedback, AT1R blockage promotes angiotensinogen and AT2R stimulation [25,48]. This balance between AT1R and AT2R is important for maintaining a normal physiological environment. Therefore, its perturbation, specifically via the AngII/AT1R connection, results in a slew of potentially harmful consequences on the endothelium, inflammation, and coagulation, in addition to the well-known vasoconstrictive effects in the brain that may potentiate the post-acute COVID symptoms. Since the interactions between AngII and AT2R and Ang II and MasR operate as a vital “protective arm” to balance these effects [57], ACE2 is considered as a key component of the anti-RAS system in the genesis and prevention of illness.
Positron emission tomography (PET) of long COVID patients, with persistent complaints at least three weeks after the onset of symptoms of acute infection, showed hypometabolism in their bilateral rectal/orbital gyrus (containing the olfactory gyrus), right temporal lobe (amygdala and hippocampus extending to the right thalamus), bilateral pons/medulla brainstem, and bilateral cerebellum. Interestingly, the metabolism of the frontal cluster (containing the olfactory gyrus) was worse in subjects receiving ACE inhibitors for high blood pressure, but better in subjects who received nasal decongestant spray [58]. These findings could point to the involvement of the ACE2 receptor in SARS-CoV-2 neurotropism, particularly in the olfactory bulb. This is likely due to the propagation pathway from the nose to the olfactory bulb, where the ACE2 receptor is strongly expressed, which has been hypothesized for various coronaviruses [59]. Cerebellar hypometabolism results in several symptoms including hyposmia/anosmia, memory/cognitive impairment, which is concordant with the involvement of this region in executive functions and working memory [60,61]. In addition, hypometabolism in the frontal cortex, brainstem, and cerebellum is associated with pain symptoms [62], particularly in fibromyalgia patients [63]. Other disorders have been linked to hypometabolism of the brainstem and cerebellum, such as insomnia and dysautonomia [64]. Taken together, the hypometabolism detected in brain regions of long COVID patients explain some of the persistent symptoms. The relationship between PET hypometabolism and the duration after the onset of the acute infection symptoms could suggest a link with the clinical severity. A more severe clinical profile is associated with a more severe PET hypometabolism and a longer duration of symptoms. Therefore, tracking functional brain activity can serve as a cerebral biomarker tool to classify long COVID patients regarding the severity of their symptoms and to distinguish affected patients from healthy subjects [58]. Also, these findings give insight to act quickly and restore brain metabolism in these patients before more severe diseases develop, such as Alzheimer’s disease (AD), which involves the severe reduction of the cerebral metabolic rate for glucose [65].
This entry is adapted from the peer-reviewed paper 10.3390/molecules27092903
This entry is offline, you can click here to edit this entry!
