Cytokines are important molecular players in cancer development, progression, and potential targets for treatment. Despite being small and overlooked, research has revealed that cytokines influence cancer biology in multiple ways. Cytokines are often found to contribute to immune function, cell damage, inflammation, angiogenesis, metastasis, and several other cellular processes important to tumor survival. Cytokines have also proven to have powerful effects on complex tumor microenvironment molecular biology and microbiology. Angiogenesis, also termed neovascularization, is blood vessel development from pre-existing vasculature. It is regulated by a careful balance of pro- and anti-angiogenic factors. In this paper, we will discuss the role of several cytokines in angiogenesis.
- angiogenesis
- cytokines
- inflammation
- tumor microenvironment
1. Introduction
2. Cytokines Drive Angiogenesis
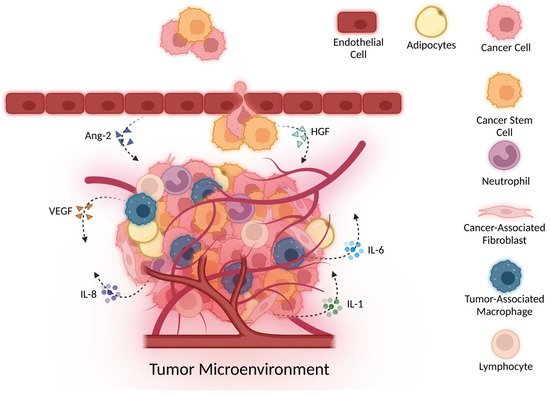
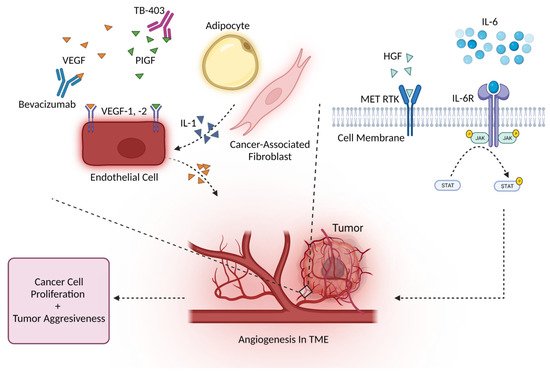
2.1. Angiogenesis and the VEGF Family
| Cytokine | Intervention/Treatment | Phase | Clinical Trial |
|---|---|---|---|
| TGF-β | Galunisertib + nivolumab Galunisertib + durvalumab Bintrafusp alfa + radiation therapy Fresolimumab + radiotherapy |
Phase 1, 2 Phase 1 Phase 1 Phase 1, 2 |
NCT02423343 NCT02734160 NCT03524170 NCT02581787 |
| TNF-α | Nivolumab + ipilimumab + certolizumab or infliximab L19 TNF-α + doxorubicin |
Phase 1 Phase 1 |
NCT03293784 NCT02076620 |
| IL-2 | NKTR-214 + pembrolizumab NKTR-214 + nivolumab NKTR-214 + nivolumab + ipilimumab aldesleukin Aldesleukin + bevacizumab Atezolizumab + cergutuzumab amunaleukin RO6874281 + trastuzumab + cetuximab |
Phase 1, 2 Phase 1 Phase 2 Phase 2 Phase 4 Phase 2 Phase 1 Phase 1 |
NCT03138889 NCT02983045 NCT03282344 NCT00006864 NCT00853021 NCT02350673 NCT02627274 |
| IL-10 | Pegilodecakin + FOLFOX | Phase 3 | NCT02923921 |
| IL-15 | N-803 rhIL-15 + NK cell infusion N-803 + aNK (NK-92) |
Phase 2 Phase 1 Phase 2 |
NCT02989844 NCT01875601 NCT02465957 |
| IL-12 | Electroporated plasmid + IL-12p DNA Pembrolizumab + pIL-12 |
n/a Phase 2 Phase 2 |
NCT00323206 NCT02345330 NCT02493361 |
| IL-8 | BMS-986253 + nivolumab or nivolumab + ipilimumab | Phase 1, 2 | NCT03400332 |
| VEGF | Bevacizumab + atezolizumab or sunitinib | Phase 2 | NCT01984242 |
| CSF-1 | APX005M + cabiralizumab + nivolumab pexidartinib + durvalumab LY3022855 + durvalumab or tremelimumab |
Phase 1 Phase 1 Phase 1 |
NCT03502330 NCT02777710 NCT02718911 |
| GM-CSF | Docetaxel and GM-CSF | Phase 2 | NCT00488982 |
| PIGF | TB-403 | Phase 1 | NCT02748135 |
2.2. Angiogenesis and Other Major Cytokines
3. Conclusion
Due to cancer’s global impact, understanding how cancer develops and progresses is critical. Cytokines are heavily involved in regulating several cancer developmental processes. Cytokines have profound effects on cells and may lead to pro- or anti-tumor activity, depending on environmental conditions and the presence of other cytokines. Overall, studies have shown that cytokines are powerful molecular players that often control angiogenesis. The impact and therapeutic potential of cytokines are beginning to be uncovered through already existing and developing cytokine-targeted therapies. In this review, we highlighted only a few cytokines involved in angiogenesis. Further research focusing on the intricate relationship between angiogenesis and cytokines will be required to increase our understanding of how cancer develops. Cytokine research will continue to play a key role in revealing the molecular mechanisms behind cancer development and angiogenesis.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2021, 71, 209–249.
- Hanahan, D. Hallmarks of Cancer: New DimensionsHallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46.
- Mascaux, C.; Angelova, M.; Vasaturo, A.; Beane, J.; Hijazi, K.; Anthoine, G.; Buttard, B.; Rothe, F.; Willard-Gallo, K.; Haller, A.; et al. Immune evasion before tumour invasion in early lung squamous carcinogenesis. Nature. 2019, 571, 570–575.
- Briukhovetska, D.; Dörr, J.; Endres, S.; Libby, P.; Dinarello, C.A.; Kobold, S. Interleukins in cancer: from biology to therapy. Nat. Rev. Cancer. 2021, 21, 481–499.
- Qiu, Y.; Su, M.; Liu, L.; Tang, Y.; Pan, Y.; Sun, J. Clinical Application of Cytokines in Cancer Immunotherapy. Drug Des. Devel. Ther. 2021, 15, 2269.
- Chen, C.; Gao, F.H. Th17 Cells Paradoxical Roles in Melanoma and Potential Application in Immunotherapy. Front. Immunol. 2019, 10, 187.
- Pradhan, R.; Chatterjee, S.; Hembram, K.C.; Sethy, C.; Mandal, M.; Kundu, C.N. Nano formulated Resveratrol inhibits metastasis and angiogenesis by reducing inflammatory cytokines in oral cancer cells by targeting tumor associated macrophages. J. Nutr. Biochem. 2021, 92, 108624.
- Bhat, S.M.; Badiger, V.A.; Vasishta, S.; Chakraborty, J.; Prasad, S.; Ghosh, S.; Joshi, M.B. 3D tumor angiogenesis models: recent advances and challenges. J. Cancer Res. Clin. Oncol. 2021, 147, 3477.
- Otrock, Z.K.; Mahfouz, R.A.R.; Makarem, J.A.; Shamseddine, A.I. Understanding the biology of angiogenesis: Review of the most important molecular mechanisms. Blood Cells, Mol. Dis. 2007, 39, 212–220.
- Siemann, D.W. The Unique Characteristics of Tumor Vasculature and Preclinical Evidence for its Selective Disruption by Tumor-Vascular Disrupting Agents. Cancer Treat. Rev. 2011, 37, 63.
- Konerding, M.A.; Fait, E.; Gaumann, A. 3D microvascular architecture of pre-cancerous lesions and invasive carcinomas of the colon. Br. J. Cancer. 2001, 84, 1354.
- Riabov, V.; Gudima, A.; Wang, N.; Mickley, A.; Orekhov, A.; Kzhyshkowska, J. Role of tumor associated macrophages in tumor angiogenesis and lymphangiogenesis. Front. Physiol. 2014, 5.
- Wang, F.T.; Sun, W.E.I.; Zhang, J.T.; Fan, Y.Z. Cancer-associated fibroblast regulation of tumor neo-angiogenesis as a therapeutic target in cancer. Oncol. Lett. 2019, 17, 3055.
- Masucci, M.T.; Minopoli, M.; Carriero, M.V. Tumor Associated Neutrophils. Their Role in Tumorigenesis, Metastasis, Prognosis and Therapy. Front. Oncol. 2019, 9, 1146.
- Hida, K.; Maishi, N.; Torii, C.; Hida, Y. Tumor angiogenesis—characteristics of tumor endothelial cells. Int. J. Clin. Oncol. 2016, 21, 206–212.
- Aras, S.; Raza Zaidi, M. TAMeless traitors: macrophages in cancer progression and metastasis. Br. J. Cancer 2017, 117, 1583.
- Uemura, A.; Fruttiger, M.; D’Amore, P.A.; De Falco, S.; Joussen, A.M.; Sennlaub, F.; Brunck, L.R.; Johnson, K.T.; Lambrou, G.N.; Rittenhouse, K.D.; et al. VEGFR1 signaling in retinal angiogenesis and microinflammation. Prog. Retin. Eye Res. 2021, 84, 100954.
- Joshi, S.; Singh, A.R.; Zulcic, M.; Durden, D.L. A Macrophage-Dominant PI3K Isoform Controls Hypoxia-Induced HIF1α and HIF2α Stability and Tumor Growth, Angiogenesis, and Metastasis. Mol. Cancer Res. 2014, 12, 1520–1531.
- Fu, R.; Han, C.F.; Ni, T.; Di, L.; Liu, L.J.; Lv, W.C.; Bi, Y.R.; Jiang, N.; He, Y.; Li, H.M.; et al. A ZEB1/p53 signaling axis in stromal fibroblasts promotes mammary epithelial tumours. Nat. Commun. 2019, 10, 3210.
- Ghafouri, S.; Burkenroad, A.; Pantuck, M.; Almomani, B.; Stefanoudakis, D.; Shen, J.; Drakaki, A. VEGF inhibition in urothelial cancer: the past, present and future. World J. Urol. 2021, 39, 741–749.
- Matsumoto-Okazaki, Y.; Yamane, J.; Kajiya, K. Real-time imaging of interaction between macrophages and lymphatic vessels in an in vitro model to study inflammatory resolution. J. Dermatol. Sci. 2015, 77, 76–79.
- Werchau, S.; Toberer, F.; Enk, A.; Dammann, R.; Helmbold, P. Merkel cell carcinoma induces lymphatic microvessel formation. J. Am. Acad. Dermatol. 2012, 67, 215–225.
- Itoh, T.; Tanioka, M.; Yoshida, H.; Yoshioka, T.; Nishimoto, H.; Itohara, S. Reduced Angiogenesis and Tumor Progression in Gelatinase A-deficient Mice. Cancer Res. 1998, 58, 1048-1051.
- Fang, J.; Shing, Y.; Wiederschain, D.; Yan, L.; Butterfield, C.; Jackson, G.; Harper, J.; Tamvakopoulos, G.; Moses, M.A. Matrix metalloproteinase-2 is required for the switch to the angiogenic phenotype in a tumor model. Proc. Natl. Acad. Sci. U. S. A. 2000, 97, 3884.
- Vu, T.H.; Shipley, J.M.; Bergers, G.; Berger, J.E.; Helms, J.A.; Hanahan, D.; Shapiro, S.D.; Senior, R.M.; Werb, Z. MMP-9/Gelatinase B Is a Key Regulator of Growth Plate Angiogenesis and Apoptosis of Hypertrophic Chondrocytes. Cell. 1998, 93, 411.
- Zhou, Z.; Apte, S.S.; Soininen, R.; Cao, R.; Baaklini, G.Y.; Rauser, R.W.; Wang, J.; Cao, Y.; Tryggvason, K. Impaired endochondral ossification and angiogenesis in mice deficient in membrane-type matrix metalloproteinase I. Proc. Natl. Acad. Sci. U. S. A. 2000, 97, 4052.
- Huang, S.; Van Arsdall, M.; Tedjarati, S.; McCarty, M.; Wu, W.; Langley, R.; Fidler, I.J. Contributions of Stromal Metalloproteinase-9 to Angiogenesis and Growth of Human Ovarian Carcinoma in Mice. JNCI J. Natl. Cancer Inst. 2002, 94, 1134–1142.
- Chetty, C.; Lakka, S.S.; Bhoopathi, P.; Rao, J.S. MMP-2 Alters VEGF Expression via αVβ3 Integrin-Mediated PI3K/AKT Signaling in A549 Lung Cancer Cells. Int. J. Cancer 2010, 127, 1081.
- Carey, P.; Low, E.; Harper, E.; Stack, M.S. Metalloproteinases in Ovarian Cancer. Int. J. Mol. Sci. 2021, 22, 3403.
- Das, S.; Amin, S.A.; Jha, T. Inhibitors of gelatinases (MMP-2 and MMP-9) for the management of hematological malignancies. Eur. J. Med. Chem. 2021, 223, 113623.
- Filipiak, J.; Boinska, J.; Ziołkowska, K.; Zduńska, M.; Zarychta, E.; Rość, D. Assessment of endothelial progenitor cells, VEGF-A and SDF-1α in Hodgkin’s lymphoma. Blood Coagul. Fibrinolysis. 2021, 32, 266–272.
- Zhang, Y.; Xiang, J.; Zhu, N.; Ge, H.; Sheng, X.; Deng, S.; Chen, J.; Yu, L.; Zhou, Y.; Shen, J. Curcumin in Combination With Omacetaxine Suppress Lymphoma Cell Growth, Migration, Invasion, and Angiogenesis via Inhibition of VEGF/Akt Signaling Pathway. Front. Oncol. 2021, 11, 656045.
- Sang, W.; Zhou, H.; Qin, Y.; Shen, Z.; Yan, D.; Sun, C.; Song, X.; Ma, Y.; Tu, D.; Bian, Z.; et al. Risk stratification model based on VEGF and International Prognostic Index accurately identifies low-risk diffuse large B-cell lymphoma patients in the rituximab era. Int. J. Hematol. 2021, 114, 189–198.
- Wang, Z.; Cao, B.; Ji, P.; Yao, F. Propofol inhibits tumor angiogenesis through targeting VEGF/VEGFR and mTOR/eIF4E signaling. Biochem. Biophys. Res. Commun. 2021, 555, 13–18.
- Chang, C.Y.; Chen, P.H.; Lu, S.C.; Hsieh, M.C.; Lin, C.W.; Lee, H.M.; Jawan, B.; Kao, Y.H. Propofol-enhanced autophagy increases motility and angiogenic capacity of cultured human umbilical vascular endothelial cells. Life Sci. 2015, 142, 49–59.
- Gao, Y.; Yu, X.; Zhang, F.; Dai, J. Propofol inhibits pancreatic cancer progress under hypoxia via ADAM8. J. Hepatobiliary. Pancreat. Sci. 2019, 26, 219–226.
- Cao, H.-Y.; Tao, T.; Shen, X.-D.; Bai, L.; Wan, C.-L.; Wu, D.-P.; Li, J.-L.; Xue, S.-L. Efficiency of anti-VEGF therapy in central nervous system AML relapse: A case report and literature review. Clin. Case Reports 2022, 10, e05367.
- Ruggiero, D.; Nutile, T.; Nappo, S.; Tirozzi, A.; Bellenguez, C.; Leutenegger, A.L.; Ciullo, M. Genetics of PlGF plasma levels highlights a role of its receptors and supports the link between angiogenesis and immunity. Sci. Rep. 2021, 11, 16821.
- Cechova, M.; Chocholaty, M.; Babjuk, M.; Zima, T.; Havlova, K.; Koldova, M.; Schmidt, M.; Kalousova, M. Diagnostic and prognostic value of placental growth factor serum concentration in clear cell renal cell carcinoma. Biomed. Pap. Med. Fac. Univ. Palacky. Olomouc. Czech. Repub. 2021, 165, 375–379.
- Arezumand, R.; Mahdian, R.; Zeinali, S.; Hassanzadeh-Ghassabeh, G.; Mansouri, K.; Khanahmad, H.; Namvar-asl, N.; Rahimi, H.; Behdani, M.; Cohan, R.A.; et al. Identification and characterization of a novel nanobody against human placental growth factor to modulate angiogenesis. Mol. Immunol. 2016, 78, 183–192.
- Nikooharf, A.; Arezumand, R.; Mansouri, K.; Khoshi, A.H.; Namdar Ahmadabad, H. Development of a Recombinant Monospecific Anti-PLGF Bivalent Nanobody and Evaluation of it in Angiogenesis Modulation. Mol. Biotechnol. 2020, 62, 580–588.
- Lassen, U.; Nielsen, D.L.; Sørensen, M.; Winstedt, L.; Niskanen, T.; Stenberg, Y.; Pakola, S.; Stassen, J.M.; Glazer, S. A phase I, dose-escalation study of TB-403, a monoclonal antibody directed against PlGF, in patients with advanced solid tumours. Br. J. Cancer. 2012, 106, 678.
- Ye, X.; Gaucher, J.F.; Vidal, M.; Broussy, S. A Structural Overview of Vascular Endothelial Growth Factors Pharmacological Ligands: From Macromolecules to Designed Peptidomimetics. Molecules. 2021, 26, 6759.
- Neufeld, G.; Kessler, O. Pro-angiogenic cytokines and their role in tumor angiogenesis. Cancer Metastasis Rev. 2006, 62, 580-588.
- Moosavi, F.; Giovannetti, E.; Saso, L.; Firuzi, O. HGF/MET pathway aberrations as diagnostic, prognostic, and predictive biomarkers in human cancers. Crit. Rev. Clin. Lab. Sci. 2019, 56, 533–566.
- Mukai, S.; Yamasaki, K.; Fujii, M.; Nagai, T.; Terada, N.; Kataoka, H.; Kamoto, T. Dysregulation of Type II Transmembrane Serine Proteases and Ligand-Dependent Activation of MET in Urological Cancers. Int. J. Mol. Sci. 2020, 21, 2663.
- Tsuji, T.; Sakamori, Y.; Ozasa, H.; Yagi, Y.; Ajimizu, H.; Yasuda, Y.; Funazo, T.; Nomizo, T.; Yoshida, H.; Nagai, H.; et al. Clinical impact of high serum hepatocyte growth factor in advanced non-small cell lung cancer. Oncotarget. 2017, 8, 71805–71816.
- Toiyama, Y.; Miki, C.; Inoue, Y.; Okugawa, Y.; Tanaka, K.; Kusunoki, M. Serum hepatocyte growth factor as a prognostic marker for stage II or III colorectal cancer patients. Int. J. Cancer. 2009, 125, 1657–1662.
- Katayama, S.; Schuettfort, V.M.; Pradere, B.; Mori, K.; Mostafaei, H.; Quhal, F.; Sari Motlagh, R.; Laukhtina, E.; Grossmann, N.C.; Aydh, A.; et al. Prognostic value of hepatocyte growth factor for muscle-invasive bladder cancer. J. Cancer Res. Clin. Oncol. 2022, 21, 1–12.
- Gao, L.-M.; Wang, F.; Zheng, Y.; Fu, Z.-Z.; Zheng, L.; Chen, L.-L. Roles of Fibroblast Activation Protein and Hepatocyte Growth Factor Expressions in Angiogenesis and Metastasis of Gastric Cancer. Pathol. Oncol. Res. 2019, 25, 369–376.
- Faiella, A.; Riccardi, F.; Cartenì, G.; Chiurazzi, M.; Onofrio, L. The Emerging Role of c-Met in Carcinogenesis and Clinical Implications as a Possible Therapeutic Target. J. Oncol. 2022, 2022, 1–12.
- Zhang, J.; Veeramachaneni, N. Targeting interleukin-1β and inflammation in lung cancer. Biomark. Res. 2022, 10, 5.
- Zhang, W.; Borcherding, N.; Kolb, R. IL-1 Signaling in Tumor Microenvironment. Adv. Exp. Med. Biol. 2020, 1240, 1–23.
- Carmi, Y.; Dotan, S.; Rider, P.; Kaplanov, I.; White, M.R.; Baron, R.; Abutbul, S.; Huszar, M.; Dinarello, C.A.; Apte, R.N.; et al. The Role of IL-1β in the Early Tumor Cell–Induced Angiogenic Response. J. Immunol. 2013, 190, 3500–3509.
- Voronov, E.; Shouval, D.S.; Krelin, Y.; Cagnano, E.; Benharroch, D.; Iwakura, Y.; Dinarello, C.A.; Apte, R.N. IL-1 is required for tumor invasiveness and angiogenesis. Proc. Natl. Acad. Sci. U. S. A. 2003, 100, 2645.
- Young, H.L.; Rowling, E.J.; Bugatti, M.; Giurisato, E.; Luheshi, N.; Arozarena, I.; Acosta, J.C.; Kamarashev, J.; Frederick, D.T.; Cooper, Z.A.; et al. An adaptive signaling network in melanoma inflammatory niches confers tolerance to MAPK signaling inhibition. J. Exp. Med. 2017, 214, 1691.
- Mechelke, T.; Wittig, F.; Ramer, R.; Hinz, B. Interleukin-1β Induces Tissue Factor Expression in A549 Cells via EGFR-Dependent and -Independent Mechanisms. Int. J. Mol. Sci. 2021, 22, 6606.
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131.
- Salgado, R.; Vermeulen, P.B.; Benoy, I.; Weytjens, R.; Huget, P.; Van Marck, E.; Dirix, L.Y. Platelet number and interleukin-6 correlate with VEGF but not with bFGF serum levels of advanced cancer patients. Br. J. Cancer. 1999, 80, 892–897.
- Xia, T.; Li, J.; Ren, X.; Liu, C.; Sun, C. Research progress of phenolic compounds regulating IL-6 to exert antitumor effects. Phyther. Res. 2021, 35, 6720–6734.
- Xu, J.; Lin, H.; Wu, G.; Zhu, M.; Li, M. IL-6/STAT3 Is a Promising Therapeutic Target for Hepatocellular Carcinoma. Front. Oncol. 2021, 11, 760971.
- Li, D.; Tang, J.; Gao, R.; Lan, J.; Shen, W.; Liu, Y.; Chen, Y.; Sun, H.; Yan, J.; Nie, Y.; et al. PFKFB4 promotes angiogenesis via IL-6/STAT5A/P-STAT5 signaling in breast cancer. J. Cancer. 2022, 13, 212.
- Fousek, K.; Horn, L.A.; Palena, C. Interleukin-8: A chemokine at the intersection of cancer plasticity, angiogenesis, and immune suppression. Pharmacol. Ther. 2021, 219, 107692.
- Dwyer, J.; Hebda, J.K.; Le Guelte, A.; Galan-Moya, E.M.; Smith, S.S.; Azzi, S.; Bidere, N.; Gavard, J. Glioblastoma Cell-Secreted Interleukin-8 Induces Brain Endothelial Cell Permeability via CXCR2. PLoS One. 2012, 7, 45562.
- Lee, Y.S.; Choi, I.; Ning, Y.; Kim, N.Y.; Khatchadourian, V.; Yang, D.; Chung, H.K.; Choi, D.; Labonte, M.J.; Ladner, R.D.; et al. Interleukin-8 and its receptor CXCR2 in the tumour microenvironment promote colon cancer growth, progression and metastasis. Br. J. Cancer. 2012, 106, 1833–1841.
- Lian, S.; Li, S.; Zhu, J.; Xia, Y.; Do Jung, Y. Nicotine stimulates IL-8 expression via ROS/NF-κB and ROS/MAPK/AP-1 axis in human gastric cancer cells. Toxicology. 2022, 466, 153062.
- Omi, K.; Matsuo, Y.; Ueda, G.; Aoyama, Y.; Kato, T.; Hayashi, Y.; Imafuji, H.; Saito, K.; Tsuboi, K.; Morimoto, M.; et al. Escin inhibits angiogenesis by suppressing interleukin-8 and vascular endothelial growth factor production by blocking nuclear factor-κB activation in pancreatic cancer cell lines. Oncol. Rep. 2021, 45, 55.
- Tsakogiannis, D.; Nikolakopoulou, A.; Zagouri, F.; Stratakos, G.; Syrigos, K.; Zografos, E.; Koulouris, N.; Bletsa, G. Update Overview of the Role of Angiopoietins in Lung Cancer. Medicina.. 2021, 57, 1191.
- Kim, H.K.; Yang, Y.; Byeon, S.; Jeong, Y.; Kwon, J.; Lee, K.H.; Son, S.M.; Han, H.S. E-Cadherin and Angiopoietin-2 as Potential Biomarkers for Colorectal Cancer With Peritoneal Carcinomatosis. Anticancer Res. 2021, 41, 4497–4504.
- Drebert, Z.; MacAskill, M.; Doughty-Shenton, D.; De Bosscher, K.; Bracke, M.; Hadoke, P.W.F.; Beck, I.M. Colon cancer-derived myofibroblasts increase endothelial cell migration by glucocorticoid-sensitive secretion of a pro-migratory factor. Vascul. Pharmacol. 2017, 89, 19.
- Lee, H.J.; Cho, C.-H.; Hwang, S.-J.; Choi, H.-H.; Kim, K.-T.; Ahn, S.Y.; Kim, J.-H.; Oh, J.-L.; Lee, G.M.; Koh, G.Y. Biological characterization of angiopoietin-3 and angiopoietin-4. FASEB J. 2004, 18, 1200–1208.
- Zhong, L.; Tang, L.; He, X. Angiopoietin-like 3 (ANGPTL3) drives cell proliferation, migration and angiogenesis in cervical cancer via binding to integrin alpha v beta 3. Bioengineered. 2022, 13, 2971–2980.
- Wu, Y.; Gao, J.; Liu, X. Deregulation of angiopoietin-like 4 slows ovarian cancer progression through vascular endothelial growth factor receptor 2 phosphorylation. Cancer Cell Int. 2021, 21, 171.
This entry is adapted from the peer-reviewed paper 10.3390/cancers14092178
