Cardiovascular diseases remain among the leading causes of death worldwide and sudden cardiac death (SCD) accounts for ~25% of these deaths. Despite its epidemiologic relevance, there are very few diagnostic strategies available useful to prevent SCD mainly focused on patients already affected by specific cardiovascular diseases. Unfortunately, most of these parameters exhibit poor positive predictive accuracy. Moreover, there is also a need to identify parameters to stratify the risk of SCD among otherwise healthy subjects.
- sudden cardiac death
- disease
- cardiac magnetic resonance
- echocardiography
1. Introduction
Echocardiography is the first and the most commonly used cardiac imaging technique. Compared to cardiac magnetic resonance and cardiac computed tomography, echocardiography is an inexpensive, rapid, and readily available imaging modality. Echocardiographic imaging of patients with ventricular arrhythmias facilitates the identification (or exclusion) of structural heart disease. Furthermore, echocardiography performed in patients who are exercising or responding to pharmacological stress can be applied to a selected group of patients with VAs triggered by ischemia [1][2].
2. Echocardiography
2.1 Left Ventricular Hypertrophy (LVH)
2.2. Global and Segmental Longitudinal Strain
2.3. Mechanical Dispersion
3. Cardiac Magnetic Resonance (CMR)
| CMR | Pathophysiologic Background | Clinical Setting | Information |
|---|---|---|---|
|
LGE |
Fibrosis |
ICM, NICM HCM, Myocarditis ARVC, LVNC Mitral valve prolapse |
Independent predictor for VA and SCD |
|
T1 and ECV |
Tissue edema and diffuse fibrosis |
ICM, NICM HCM, Myocarditis |
Higher native T1 values associated with VA |
|
T2 |
Myocardial edema |
Myocarditis |
Abnormal T2 mapping is involved in predicting major adverse events including cardiac death |
|
LVEF |
Left ventricular systolic function |
ICM, NICM Myocarditis, ARVC LVNC |
LV systolic dysfunction is associated with an increased risk of SCD |
|
RVEF |
Right ventricular systolic function |
ARVDC |
Overall increase in VA in RV dysfunction |
|
Strain Imaging and MD |
Myocardial deformation and function |
ICM, NICM |
Impaired strain associated with SCD |
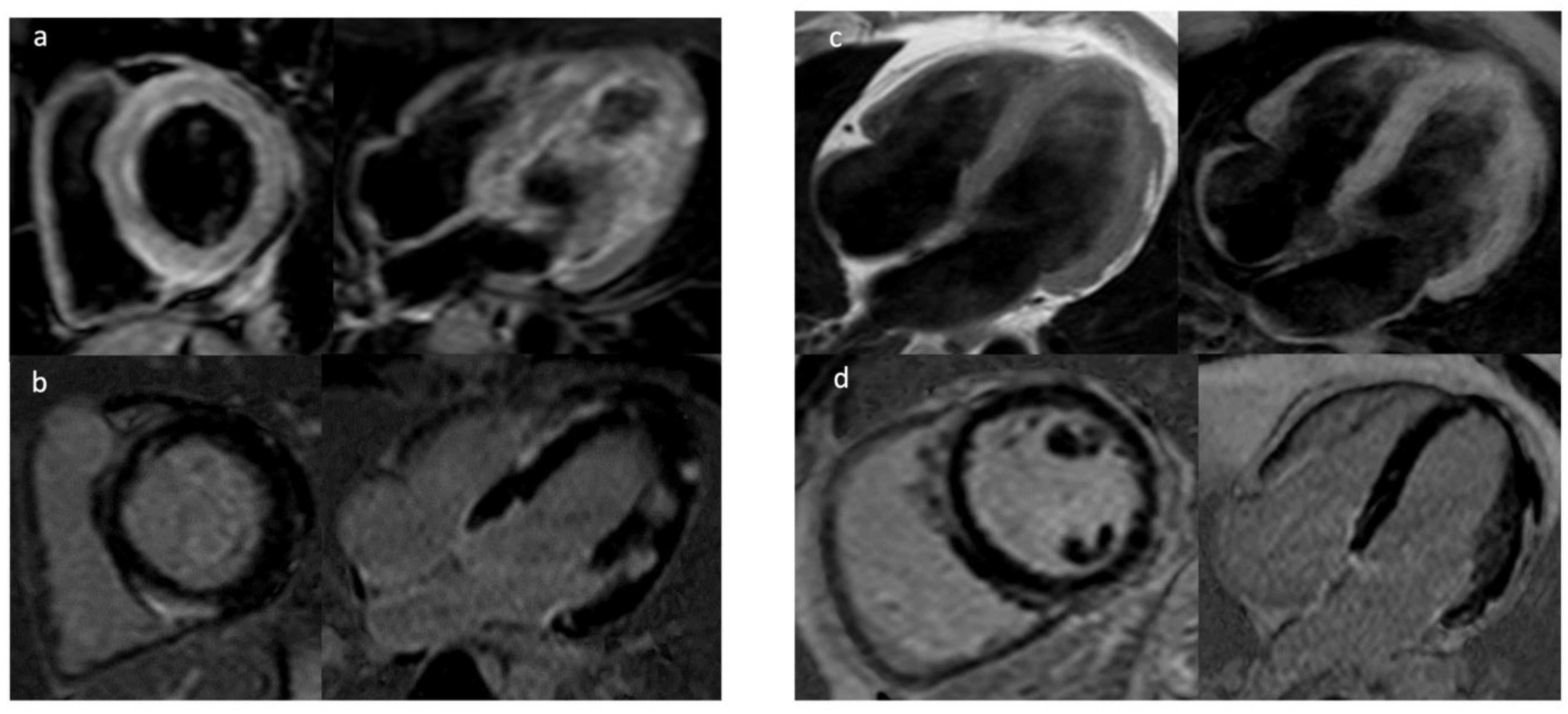
3.1. Late Gadolinium Enhancement
3.2. Mapping Techniques and Extracellular Volume
3.3. Feature Tracking (FT)
3.4. CMR in Mitral Valve Prolapse
This entry is adapted from the peer-reviewed paper 10.3390/jcm11061519
References
- Al-Khatib, S.M.; Stevenson, W.G.; Ackerman, M.J.; Al Bryant, W.J.; Callans, D.J.; Curtis, A.B.; Deal, B.J.; Dickfeld, T.; Field, M.E.; Fonarow, G.C.; et al. 2017 AHA/ACC/HRS Guideline for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation 2018, 138, 210–271.
- Priori, S.G.; Blomström-Lundqvist, C. 2015 European Society of Cardiology Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death summarized by co-chairs. Eur. Heart J. 2015, 36, 2757–2759.
- Konety, S.H.; Koene, R.J.; Norby, F.L.; Wilsdon, T.; Alonso, A.; Siscovick, D.; Sotoodehnia, N.; Gottdiener, J.; Fox, E.R.; Chen, L.Y.; et al. Echocardiographic Predictors of Sudden Cardiac Death: The Atherosclerosis Risk in Communities Study and Cardiovascular Health Study. Circ. Cardiovasc. Imaging. 2016, 9, e004431.
- Laukkanen, J.A.; Khan, H.; Kurl, S.; Willeit, P.; Karppi, J.; Ronkainen, K.; Di Angelantonio, E. Left ventricular mass and the risk of sudden cardiac death: A population-based study. J. Am. Heart Assoc. 2014, 3, e001285.
- Verheule, S.; Schotten, U. Electrophysiological Consequences of Cardiac Fibrosis. Cells 2021, 10, 3220.
- Haider, A.W.; Larson, M.G.; Benjamin, E.J.; Levy, D. Increased left ventricular mass and hypertrophy are associated with increased risk for sudden death. J. Am. Coll. Cardiol. 1998, 32, 1454–1459.
- Reinier, K.; Dervan, C.; Singh, T.; Uy-Evanado, A.; Lai, S.; Gunson, K.; Jui, J.; Chugh, S.S. Increased left ventricular mass and decreased left ventricular systolic function have independent pathways to ventricular arrhythmogenesis in coronary artery disease. Heart Rhythm 2011, 8, 1177–1182.
- Ng, A.C.; Bertini, M.; Borleffs, C.J.; Delgado, V.; Boersma, E.; Piers, S.R.; Thijssen, J.; Nucifora, G.; Shanks, M.; Ewe, S.H.; et al. Predictors of death and occurrence of appropriate implantable defibrillator therapies in patients with ischemic cardiomyopathy. Am. J. Cardiol. 2010, 106, 1566–1573.
- Ersbøll, M.; Valeur, N.; Andersen, M.J.; Mogensen, U.M.; Vinther, M.; Svendsen, J.H.; Møller, J.E.; Kisslo, J.; Velazquez, E.J.; Hassager, C.; et al. Early echocardiographic deformation analysis for the prediction of sudden cardiac death and life-threatening arrhythmias after myocardial infarction. JACC Cardiovasc. Imaging 2013, 6, 851–860.
- Biering-Sørensen, T.; Knappe, D.; Pouleur, A.C.; Claggett, B.; Wang, P.J.; Moss, A.J.; Solomon, S.D.; Kutyifa, V. Regional Longitudinal Deformation Improves Prediction of Ventricular Tachyarrhythmias in Patients With Heart Failure With Reduced Ejection Fraction: A MADIT-CRT Substudy (Multicenter Automatic Defibrillator Implantation Trial-Cardiac Resynchronization Therapy). Circ. Cardiovasc. Imaging 2017, 10, e005096.
- Wu, T.J.; Ong, J.J.; Hwang, C.; Lee, J.J.; Fishbein, M.C.; Czer, L.; Trento, A.; Blanche, C.; Kass, R.M.; Mandel, W.J.; et al. Characteristics of wave fronts during ventricular fibrillation in human hearts with dilated cardiomyopathy: Role of increased fibrosis in the generation of reentry. J. Am. Coll. Cardiol. 1998, 32, 187–196.
- Haugaa, K.H.; Grenne, B.L.; Eek, C.H.; Ersbøll, M.; Valeur, N.; Svendsen, J.H.; Florian, A.; Sjøli, B.; Brunvand, H.; Køber, L.; et al. Strain echocardiography improves risk prediction of ventricular arrhythmias after myocardial infarction. JACC Cardiovasc. Imaging 2013, 6, 841–850.
- Perry, R.; Patil, S.; Marx, C.; Horsfall, M.; Chew, D.P.; Sree Raman, K.; Daril, N.D.M.; Tiver, K.; Joseph, M.X.; Ganesan, A.N.; et al. Advanced Echocardiographic Imaging for Prediction of SCD in Moderate and Severe LV Systolic Function. JACC Cardiovasc. Imaging 2020, 13, 604–612.
- Debonnaire, P.; Thijssen, J.; Leong, D.P.; Joyce, E.; Katsanos, S.; Hoogslag, G.E.; Schalij, M.J.; Atsma, D.E.; Bax, J.J.; Delgado, V.; et al. Global longitudinal strain and left atrial volume index improve prediction of appropriate implantable cardioverter defibrillator therapy in hypertrophic cardiomyopathy patients. Int. J. Cardiovasc. Imaging 2014, 30, 549–558.
- Haland, T.F.; Almaas, V.M.; Hasselberg, N.E.; Saberniak, J.; Leren, I.S.; Hopp, E.; Edvardsen, T.; Haugaa, K.H. Strain echocardiography is related to fibrosis and ventricular arrhythmias in hypertrophic cardiomyopathy. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 613–621.
- Ermakov, S.; Gulhar, R.; Lim, L.; Bibby, D.; Fang, Q.; Nah, G.; Abraham, T.P.; Schiller, N.B.; Delling, F.N. Left ventricular mechanical dispersion predicts arrhythmic risk in mitral valve prolapse. Heart 2019, 105, 1063–1069.
- Alizade, E.; Yesin, M.; Tabakci, M.M.; Avci, A.; Bulut, M.; Acar, G.; Şimşek, Z.; Izci, S.; Barutçu, S.; Pala, S. Utility of speckle tracking echocardiography imaging in patients with asymptomatic and symptomatic arrhythmogenic right ventricular cardiomyopathy. Echocardiography 2016, 33, 1683–1688.
- Sarvari, S.I.; Haugaa, K.H.; Anfinsen, O.G.; Leren, T.P.; Smiseth, O.A.; Kongsgaard, E.; Amlie, J.P.; Edvardsen, T. Right ventricular mechanical dispersion is related to malignant arrhythmias: A study of patients with arrhythmogenic right ventricular cardiomyopathy and subclinical right ventricular dysfunction. Eur. Heart J. 2011, 32, 1089–1096.
- Gulati, A.; Jabbour, A.; Ismail, T.F.; Guha, K.; Khwaja, J.; Raza, S.; Morarji, K.; Brown, T.D.; Ismail, N.A.; Dweck, M.R.; et al. Association of fibrosis with mortality and sudden cardiac death in patients with nonischemic dilated cardiomyopathy. JAMA 2013, 309, 896–908.
- Disertori, M.; Rigoni, M.; Pace, N.; Casolo, G.; Masè, M.; Gonzini, L.; Lucci, D.; Nollo, G.; Ravelli, F. Myocardial Fibrosis Assessment by LGE Is a Powerful Predictor of Ventricular Tachyarrhythmias in Ischemic and Nonischemic LV Dysfunction: A Meta-Analysis. JACC Cardiovasc. Imaging 2016, 9, 1046–1055.
- Halliday, B.P.; Gulati, A.; Ali, A.; Guha, K.; Newsome, S.; Arzanauskaite, M.; Vassiliou, V.S.; Lota, A.; Izgi, C.; Tayal, U.; et al. Association Between Midwall Late Gadolinium Enhancement and Sudden Cardiac Death in Patients With Dilated Cardiomyopathy and Mild and Moderate Left Ventricular Systolic Dysfunction. Circulation 2017, 135, 2106–2115.
- Di Marco, A.; Anguera, I.; Schmitt, M.; Klem, I.; Neilan, T.G.; White, J.A.; Sramko, M.; Masci, P.G.; Barison, A.; Mckenna, P.; et al. Late Gadolinium Enhancement and the Risk for Ventricular Arrhythmias or Sudden Death in Dilated Cardiomyopathy: Systematic Review and Meta-Analysis. JACC Heart Fail. 2017, 5, 28–38.
- Piers, S.R.; Everaerts, K.; van der Geest, R.J.; Hazebroek, M.R.; Siebelink, H.M.; Pison, L.A.; Schalij, M.J.; Bekkers, S.C.; Heymans, S.; Zeppenfeld, K. Myocardial scar predicts monomorphic ventricular tachycardia but not polymorphic ventricular tachycardia or ventricular fibrillation in nonischemic dilated cardiomyopathy. Heart Rhythm 2015, 12, 2106–2114.
- Assomull, R.G.; Prasad, S.K.; Lyne, J.; Smith, G.; Burman, E.D.; Khan, M.; Sheppard, M.N.; Poole-Wilson, P.A.; Pennell, D.J. Cardiovascular magnetic resonance, fibrosis, and prognosis in dilated cardiomyopathy. J. Am. Coll. Cardiol. 2006, 48, 1977–1985.
- Gräni, C.; Eichhorn, C.; Bière, L.; Murthy, V.L.; Agarwal, V.; Kaneko, K.; Cuddy, S.; Aghayev, A.; Steigner, M.; Blankstein, R.; et al. Prognostic Value of Cardiac Magnetic Resonance Tissue Characterization in Risk Stratifying Patients With Suspected Myocarditis. J. Am. Coll. Cardiol. 2017, 70, 1964–1976.
- Cheong, B.Y.; Muthupillai, R.; Wilson, J.M.; Sung, A.; Huber, S.; Amin, S.; Elayda, M.A.; Lee, V.V.; Flamm, S.D. Prognostic significance of delayed-enhancement magnetic resonance imaging: Survival of 857 patients with and without left ventricular dysfunction. Circulation 2009, 120, 2069–2076.
- Klem, I.; Weinsaft, J.W.; Bahnson, T.D.; Hegland, D.; Kim, H.W.; Hayes, B.; Parker, M.A.; Judd, R.M.; Kim, R.J. Assessment of myocardial scarring improves risk stratification in patients evaluated for cardiac defibrillator implantation. J. Am. Coll. Cardiol. 2012, 60, 408–420.
- Kwong, R.Y.; Chan, A.K.; Brown, K.A.; Chan, C.W.; Reynolds, H.G.; Tsang, S.; Davis, R.B. Impact of unrecognized myocardial scar detected by cardiac magnetic resonance imaging on event-free survival in patients presenting with signs or symptoms of coronary artery disease. Circulation 2006, 113, 2733–2743.
- Grün, S.; Schumm, J.; Greulich, S.; Wagner, A.; Schneider, S.; Bruder, O.; Kispert, E.M.; Hill, S.; Ong, P.; Klingel, K.; et al. Long-term follow-up of biopsy-proven viral myocarditis: Predictors of mortality and incomplete recovery. J. Am. Coll. Cardiol. 2012, 59, 1604–1615.
- Aquaro, G.D.; Perfetti, M.; Camastra, G.; Monti, L.; Dellegrottaglie, S.; Moro, C.; Pepe, A.; Todiere, G.; Lanzillo, C.; Scatteia, A.; et al. Cardiac MR With Late Gadolinium Enhancement in Acute Myocarditis With Preserved Systolic Function: ITAMY Study. J. Am. Coll. Cardiol. 2017, 70, 1977–1987.
- Halliday, B.P.; Baksi, A.J.; Gulati, A.; Ali, A.; Newsome, S.; Izgi, C.; Arzanauskaite, M.; Lota, A.; Tayal, U.; Vassiliou, V.S.; et al. Outcome in Dilated Cardiomyopathy Related to the Extent, Location, and Pattern of Late Gadolinium Enhancement. JACC Cardiovasc. Imaging 2019, 12, 1645–1655.
- Schuleri, K.H.; Centola, M.; Evers, K.S.; Zviman, A.; Evers, R.; Lima, J.A.; Lardo, A.C. Cardiovascular magnetic resonance characterization of peri-infarct zone remodeling following myocardial infarction. J. Cardiovasc. Magn. Reson. 2012, 14, 24.
- Demirel, F.; Adiyaman, A.; Timmer, J.R.; Dambrink, J.H.; Kok, M.; Boeve, W.J.; Elvan, A. Myocardial scar characteristics based on cardiac magnetic resonance imaging is associated with ventricular tachyarrhythmia in patients with ischemic cardiomyopathy. Int. J. Cardiol. 2014, 177, 392–399.
- Kwon, D.H.; Halley, C.M.; Carrigan, T.P.; Zysek, V.; Popovic, Z.B.; Setser, R.; Schoenhagen, P.; Starling, R.C.; Flamm, S.D.; Desai, M.Y. Extent of left ventricular scar predicts outcomes in ischemic cardiomyopathy patients with significantly reduced systolic function: A delayed hyperenhancement cardiac magnetic resonance study. JACC Cardiovasc. Imaging 2009, 2, 34–44.
- Gao, P.; Yee, R.; Gula, L.; Krahn, A.D.; Skanes, A.; Leong-Sit, P.; Klein, G.J.; Stirrat, J.; Fine, N.; Pallaveshi, L.; et al. Prediction of arrhythmic events in ischemic and dilated cardiomyopathy patients referred for implantable cardiac defibrillator: Evaluation of multiple scar quantification measures for late gadolinium enhancement magnetic resonance imaging. Circ. Cardiovasc. Imaging 2012, 5, 448–456.
- Schmidt, A.; Azevedo, C.F.; Cheng, A.; Gupta, S.N.; Bluemke, D.A.; Foo, T.K.; Gerstenblith, G.; Weiss, R.G.; Marbán, E.; Tomaselli, G.F.; et al. Infarct tissue heterogeneity by magnetic resonance imaging identifies enhanced cardiac arrhythmia susceptibility in patients with left ventricular dysfunction. Circulation 2007, 115, 2006–2014.
- Jablonowski, R.; Chaudhry, U.; van der Pals, J.; Engblom, H.; Arheden, H.; Heiberg, E.; Wu, K.C.; Borgquist, R.; Carlsson, M. Cardiovascular Magnetic Resonance to Predict Appropriate Implantable Cardioverter Defibrillator Therapy in Ischemic and Nonischemic Cardiomyopathy Patients Using Late Gadolinium Enhancement Border Zone: Comparison of Four Analysis Methods. Circ. Cardiovasc. Imaging 2017, 10, e006105.
- Yan, A.T.; Shayne, A.J.; Brown, K.A.; Gupta, S.N.; Chan, C.W.; Luu, T.M.; Di Carli, M.F.; Reynolds, H.G.; Stevenson, W.G.; Kwong, R.Y. Characterization of the peri-infarct zone by contrast-enhanced cardiac magnetic resonance imaging is a powerful predictor of post-myocardial infarction mortality. Circulation 2006, 114, 32–39.
- Moon, J.C.; Reed, E.; Sheppard, M.N.; Elkington, A.G.; Ho, S.Y.; Burke, M.; Petrou, M.; Pennell, D.J. The histologic basis of late gadolinium enhancement cardiovascular magnetic resonance in hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 2004, 43, 2260–2264.
- Chan, R.H.; Maron, B.J.; Olivotto, I.; Pencina, M.J.; Assenza, G.E.; Haas, T.; Lesser, J.R.; Gruner, C.; Crean, A.M.; Rakowski, H.; et al. Prognostic value of quantitative contrast-enhanced cardiovascular magnetic resonance for the evaluation of sudden death risk in patients with hypertrophic cardiomyopathy. Circulation 2014, 130, 484–495.
- Chen, Z.; Sohal, M.; Voigt, T.; Sammut, E.; Tobon-Gomez, C.; Child, N.; Jackson, T.; Shetty, A.; Bostock, J.; Cooklin, M.; et al. Myocardial tissue characterization by cardiac magnetic resonance imaging using T1 mapping predicts ventricular arrhythmia in ischemic and non-ischemic cardiomyopathy patients with implantable cardioverter-defibrillators. Heart Rhythm 2015, 12, 792–801.
- Flett, A.S.; Hayward, M.P.; Ashworth, M.T.; Hansen, M.S.; Taylor, A.M.; Elliott, P.M.; McGregor, C.; Moon, J.C. Equilibrium contrast cardiovascular magnetic resonance for the measurement of diffuse myocardial fibrosis: Preliminary validation in humans. Circulation 2010, 122, 138–144.
- Spieker, M.; Haberkorn, S.; Gastl, M.; Behm, P.; Katsianos, S.; Horn, P.; Jacoby, C.; Schnackenburg, B.; Reinecke, P.; Kelm, M.; et al. Abnormal T2 mapping cardiovascular magnetic resonance correlates with adverse clinical outcome in patients with suspected acute myocarditis. J. Cardiovasc. Magn. Reson. 2017, 19, 38.
- Haaf, P.; Garg, P.; Messroghli, D.R.; Broadbent, D.A.; Greenwood, J.P.; Plein, S. Cardiac T1 Mapping and Extracellular Volume (ECV) in clinical practice: A comprehensive review. J. Cardiovasc. Magn. Reson. 2016, 18, 89.
- Messroghli, D.R.; Moon, J.C.; Ferreira, V.M.; Grosse-Wortmann, L.; He, T.; Kellman, P.; Mascherbauer, J.; Nezafat, R.; Salerno, M.; Schelbert, E.B.; et al. Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2* and extracellular volume: A consensus statement by the Society for Cardiovascular Magnetic Resonance (SCMR) endorsed by the European Association for Cardiovascular Imaging (EACVI). J. Cardiovasc. Magn. Reson. 2017, 19, 75.
- Leyva, F.; Zegard, A.; Acquaye, E.; Gubran, C.; Taylor, R.; Foley, P.W.X.; Umar, F.; Patel, K.; Panting, J.; Marshall, H.; et al. Outcomes of Cardiac Resynchronization Therapy With or without Defibrillation in Patients with Nonischemic Cardiomyopathy. J. Am. Coll. Cardiol. 2017, 70, 1216–1227.
- Overhoff, D.; Ansari, U.; Hohneck, A.; Tülümen, E.; Rudic, B.; Kuschyk, J.; Lossnitzer, D.; Baumann, S.; Froelich, M.F.; Waldeck, S.; et al. Prediction of cardiac events with non-contrast magnetic resonance feature tracking in patients with ischaemic cardiomyopathy. ESC Heart Fail. 2022, 9, 574–584.
- Miller, M.A.; Dukkipati, S.R.; Turagam, M.; Liao, S.L.; Adams, D.H.; Reddy, V.Y. Arrhythmic Mitral Valve Prolapse: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2018, 72, 2904–2914.
- Basso, C.; Perazzolo Marra, M.; Rizzo, S.; De Lazzari, M.; Giorgi, B.; Cipriani, A.; Frigo, A.C.; Rigato, I.; Migliore, F.; Pilichou, K.; et al. Arrhythmic Mitral Valve Prolapse and Sudden Cardiac Death. Circulation 2015, 132, 556–566.
- Syed, F.F.; Ackerman, M.J.; McLeod, C.J.; Kapa, S.; Mulpuru, S.K.; Sriram, C.S.; Cannon, B.C.; Asirvatham, S.J.; Noseworthy, P.A. Sites of Successful Ventricular Fibrillation Ablation in Bileaflet Mitral Valve Prolapse Syndrome. Circ. Arrhythm. Electrophysiol. 2016, 9, e004005.
- Bui, A.H.; Roujol, S.; Foppa, M.; Kissinger, K.V.; Goddu, B.; Hauser, T.H.; Zimetbaum, P.J.; Ngo, L.H.; Manning, W.J.; Nezafat, R.; et al. Diffuse myocardial fibrosis in patients with mitral valve prolapse and ventricular arrhythmia. Heart 2017, 103, 204–209.
- Perazzolo Marra, M.; Basso, C.; De Lazzari, M.; Rizzo, S.; Cipriani, A.; Giorgi, B.; Lacognata, C.; Rigato, I.; Migliore, F.; Pilichou, K.; et al. Morphofunctional Abnormalities of Mitral Annulus and Arrhythmic Mitral Valve Prolapse. Circ. Cardiovasc. Imaging 2016, 9, e005030.
- Nadel, J.; Lancefield, T.; Voskoboinik, A.; Taylor, A.J. Late gadolinium enhancement identified with cardiac magnetic resonance imaging in sarcoidosis patients is associated with long-term ventricular arrhythmia and sudden cardiac death. EurHeart J. Cardiovasc. Imaging 2015, 16, 634–641.
- Ichinose, A.; Otani, H.; Oikawa, M.; Takase, K.; Saito, H.; Shimokawa, H.; Takahashi, S. MRI of cardiac sarcoidosis: Basal and subepicardial localization of myocardial lesions and their effect on left ventricular function. AJR 2008, 191, 862–869.
- Crawford, T.; Mueller, G.; Sarsam, S.; Prasitdumrong, H.; Chaiyen, N.; Gu, X.; Schuller, J.; Kron, J.; Nour, K.A.; Cheng, A.; et al. Magnetic resonance imaging for identifying patients with cardiac sarcoidosis and preserved or mildly reduced left ventricular function at risk of ventricular arrhythmias. Circ. Arrhythm. Electrophysiol. 2014, 7, 1109–1115.
- Patel, A.R.; Klein, M.R.; Chandra, S.; Spencer, K.T.; Decara, J.M.; Lang, R.M.; Burke, M.C.; Garrity, E.R.; Hogarth, D.K.; Archer, S.L.; et al. Myocardial damage in patients with sarcoidosis and preserved left ventricular systolic function: An observational study. Eur. J. Heart Fail. 2011, 13, 1231–1237.
- Smedema, J.P.; van Geuns, R.J.; Ainslie, G.; Ector, J.; Heidbuchel, H.; Crijns, H. Right ventricular involvement in cardiac sarcoidosis demonstrated with cardiac magnetic resonance. ESC HeartFail. 2017, 4, 535–544.
