The corneal surface is an essential organ necessary for vision, and its clarity must be maintained. The corneal epithelium is renewed by limbal stem cells, located in the limbus and in palisades of Vogt. Palisades of Vogt maintain the clearness of the corneal epithelium by blocking the growth of conjunctival epithelium and the invasion of blood vessels over the cornea. The limbal region can be damaged by chemical burns, physical damage (e.g., by contact lenses), congenital disease, chronic inflammation, or limbal surgeries. The degree of limbus damage is associated with the degree of limbal stem cells deficiency (partial or total). For a long time, the only treatment to restore vision was grafting part of the healthy cornea from the other eye of the patient or by transplanting a cornea from cadavers. The regenerative medicine and stem cell therapies have been applied to restore normal vision using different methodologies. The source of stem cells varies from embryonic stem cells, mesenchymal stem cells, to induced pluripotent stem cells. This review focuses on the use of oral mucosa epithelial stem cells and their use in engineering cell sheets to treat limbal stem cell deficient patients.
- cell sheet
- limbal stem cell deficiency
- tissue engineering
- clinical trial
- oral mucosa epithelial cells
A healthy cornea is essential for proper vision. This part of the eye must be kept clear to be fully functional. The cornea is divided into different parts: (1) the corneal epithelium, (2) the Bowman membrane, (3) the stroma, (4) the Descemet membrane, and (5) the corneal endothelium (Scheme 1). The corneal epithelium is constantly renewed by limbal stem cells, located in the limbus. The corneal epithelial cells completely renew in five to seven days [6]. The asymmetric division of the limbal stem cells generates a limbal stem daughter cell and a transient amplifying cell, which migrate to the central cornea. Limbal stem cells migrate toward the middle of the corneal epithelium, in an X, Y, Z direction [[1]]. During their migration, limbal stem cells differentiate until they become squamous cells and detach from the surface of the cornea [[2]].
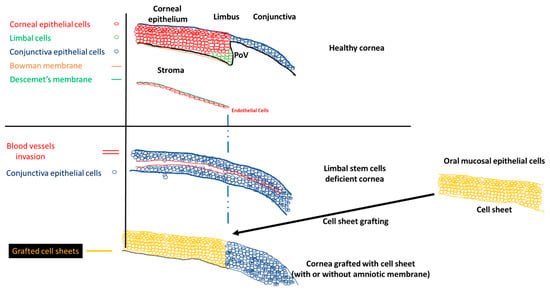
Scheme 1. Normal cornea, limbal stem cell cornea, and the grafting of the cell sheets on limbal stem cell deficient corneas to restore the corneal morphology. POV, palisades of Vogt.
The stem cell niche is precisely located at the level of palisades of Vogt, in the limbus [[3]]. Injury to the limbal niche prevents corneal epithelial cell renewal and results in the growth of conjunctival epithelium over the cornea. Conjunctival growth over the cornea is accompanied by the neovascularization of the cornea. Many studies reported that the limbal region functions as a barrier between the cornea and conjunctiva. The role of the barrier is to block the conjunctivalization and the neovascularization of the cornea [[4][5][6][7]]. Damage to this barrier leads to the development of limbal stem cell deficiency (LSCD). The process of conjunctivalization, where conjunctival epithelial cells invade and populate the corneal surface, results in neovascularization, opacification, and inflammatory cell infiltration [[8][9][10]]. This limbal stem cell deficiency leads to different levels of visual impairment, as reported by the international Limbal Stem Cell Deficiency Working Group [[8], [11],[12]]. LSCD can be caused by exogenous trauma, such as thermal burns, chemical injury (alkali burn), or endogenous eye diseases (e.g., Stevens–Johnson syndrome, ocular pemphigoid, aniridia (a genetic disorder), contact lenses, multiple surgeries, or microbial infection) [[11], [13]].
Different methodologies have been developed since 1905, when the first corneal transplantation was completed by Dr. Eduard Zirm [[14]]. Corneal transplantation is used to repair only the damaged central part of the cornea, but cannot restore the presence of limbal stem cells, which are involved in the renewal of the corneal epithelium, or be used for long term treatment of corneal epithelial defects due to limbal stem cell deficiency. Limbal stem cell transplantation is used to restore the renewal process of the corneal epithelium when the limbal stem cells are damaged and can no longer perform their duty. The curing potency of the limbal stem cell graft can be superior to the corneal transplantation. Grafting of limbal stem cells renews the central part of the damaged cornea to treat corneal epithelial defects [[15]].
No approved treatment currently exists for LSCD patients other than: (1) autologous grafting of limbal stem cells [[16][17]] and (2) allografting of limbal epithelium from a deceased donor [[18][19]]. Autologous grafts produce excellent results in treating the LSCD cornea because the risk of graft rejection from the transplant is reduced. However, this treatment has limitations: (1) this approach cannot be performed if the patient has bilateral LSCD, which is a challenging task for ophthalmologists [[20]]; (2) a risk exists of damaging the healthy cornea [9]. Donor cornea allograft treatment heavily depends on the supply of donor corneas provided by eye banks. The shortage of donor eyes is well known worldwide as a serious problem [[21]]. Even when a donor cornea is grafted onto a patient’s eye, a long-term immunosuppressant treatment to decrease the graft rejection risk is required [[22][23]].
Among the cells used for corneal recovery, oral mucosa epithelial cells are the most commonly used for in vitro, in vivo, and translational applications. Different laboratories and hospitals over the world engineered oral mucosal epithelial cell sheets to treated patients afflicted with LSCD, in clinical trials studies. Table S1 summarizes the type of cells used to treat LSCD, and the table S2 reports all the clinical trials published, treating LSCD with oral mucosal epithelial cells.
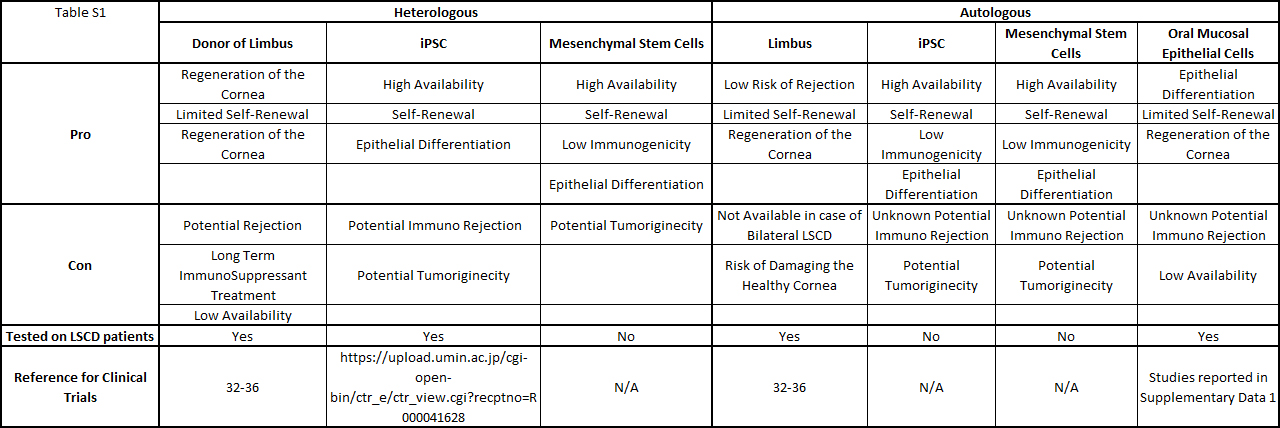
Table S1: Summary of the cells used to treat patients LSCD.
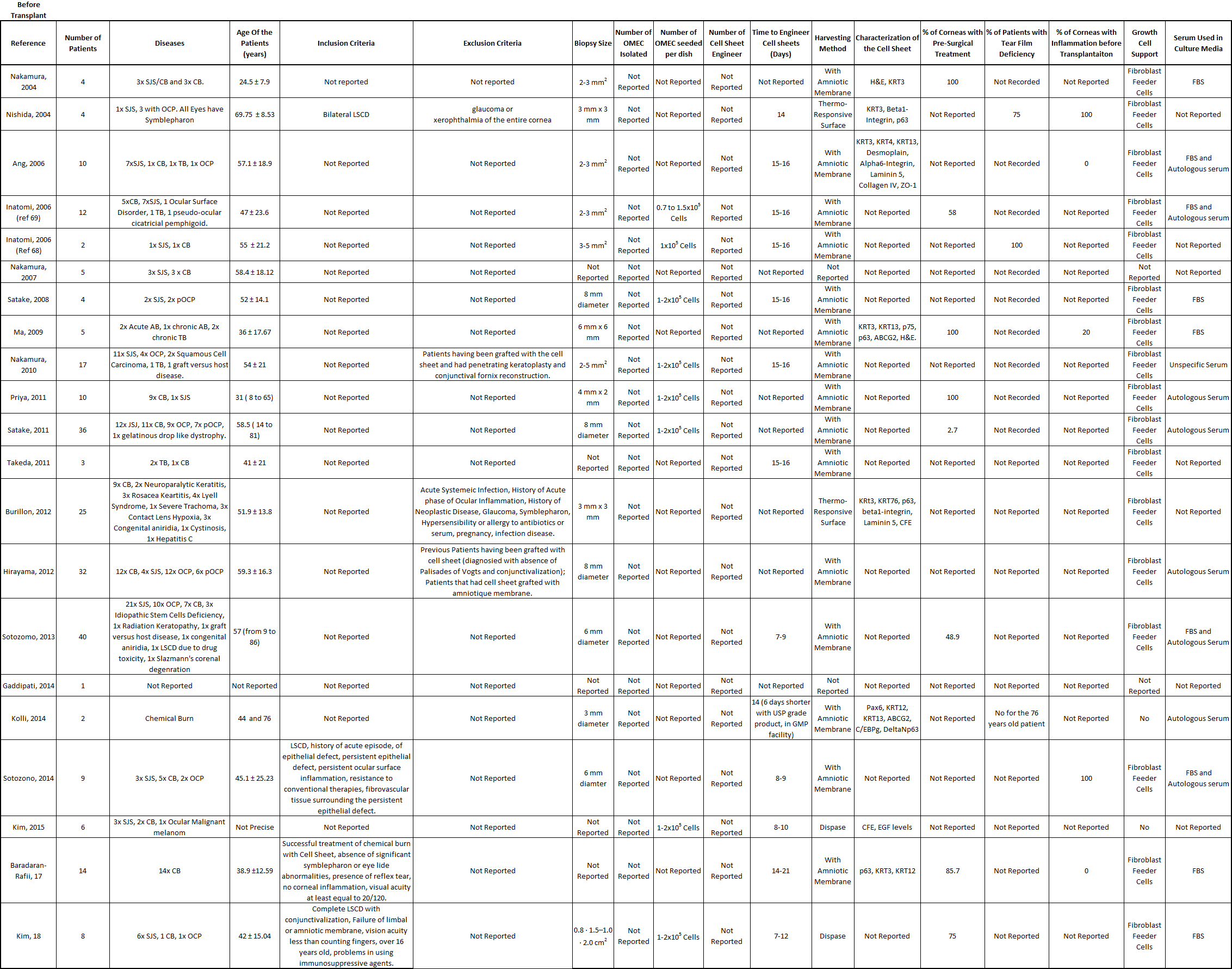
Table S2: Summarizes of data related with the patients recruited to treat LSCD in clinical trials, with oral mucosal epithelial cells.
In general, the results are very positive and encouraging, but the technology of oral mucosa epithelial cell sheet can be improved. The average time for the follow-up of the patients was around two years, with a maximum of 7.5 years, for all the clinical trials involving oral mucosal epithelial cell sheets to treat LSCD. The visual acuity improved for the majority of the patients treated with oral mucosal epithelial cell sheets. For 246 transplanted corneas, 52.8% of the corneas demonstrated an improvement, 8.2% were steady, and 2.9% deteriorated. Based on a review, the transplant of limbal stem cells was over 70% successful, and it is thought that this success rate can be increased. We think that oral mucosal epithelial cell sheet technology to treat LSCD can also be improved, because only 52.8% of the transplanted cornea resulted in improved visual acuity. Neovascularization of the grafted cornea occurred [[24][25][26]], but never grew over the cornea, indicating that cultured autologous oral mucosal epithelial cell sheet (CAOMECS) can block the neovascularization of the cornea of the healthy cornea [[27]]. The mechanism of action of the cell sheet is not well understood, but it could involve a combination of the physical barrier and the production of anti-angiogenic factors. The physical barrier function is indicated by the decrease in the corneal conjunctivalization level after the epithelium cell sheet graft, but this is not always the case [[28][29]]. Corneal opacity is another aspect of patient outcome. Corneal opacity decreased over time after a cell sheet graft in some studies [[30],[31]]. If the opacity did not improve and was persistent, penetrating or deep lamella keratoplasty (PKP and DALK, respectively) was performed [[32][33][34][35][36][37]]; in some cases, two years after the initial grafting [[28], [38]]. PKP and DALK should only be performed after cell sheet graft and once the cornea surface is stable.
A total of 55 adverse events (AEs) was reported, with only nine severe adverse events (SAEs) among the 249 treated patients. Among the 55 AEs, persistent epithelial defects (PEDs) were the most frequent (30 cases), and they were recurrent after the initial cell sheet transplantation (54.54% of the total AEs). A review explained how persistent epithelial defects are treated [[39]]. In some of the clinical studies, the PEDs resolved by themselves with the help of grafted cell sheets or conjunctival cells [[33], [40][41]]; antibiotics were used [[25]], a second reoperation was performed (oral mucosa epithelial cell sheet, amniotic membrane, allogenic corneal, or limbal stem cells transplants) [[33], [37], [40]], with a contact lens bandage [[26], [42]]; or they did not resolve [[29], [32], [43][44][45]]. Intraocular pressure was reported for 14 of the patients (5.62% of all patients), which resolved with specific treatment, such as antiglaucoma medication or carbonic anhydrase inhibitor [[28], [29]]. Infection of the cornea was detected in four patients (1.6% of the total number of patients), and the infections resolved with antibiotic treatment [[25], [29], [32], [37]]. Some other AEs were recorded, such as cataract, pain, corneal recurrence, Meibomian cyst, keratitis, symblepharon formation, drug induced allergy, and liver dysfunction, which resolved after stopping a systemic drug treatment [[32],[35], [43]]. Nine SAEs were reported: two corneal perforations, where one cornea healed with a small patch graft [36], and the patient in the other study was withdrawn [[43]]; seven cell sheets were rejected [[33], [35], [37], [43]]. Follow up and reports of adverse events are reported in the table S3.
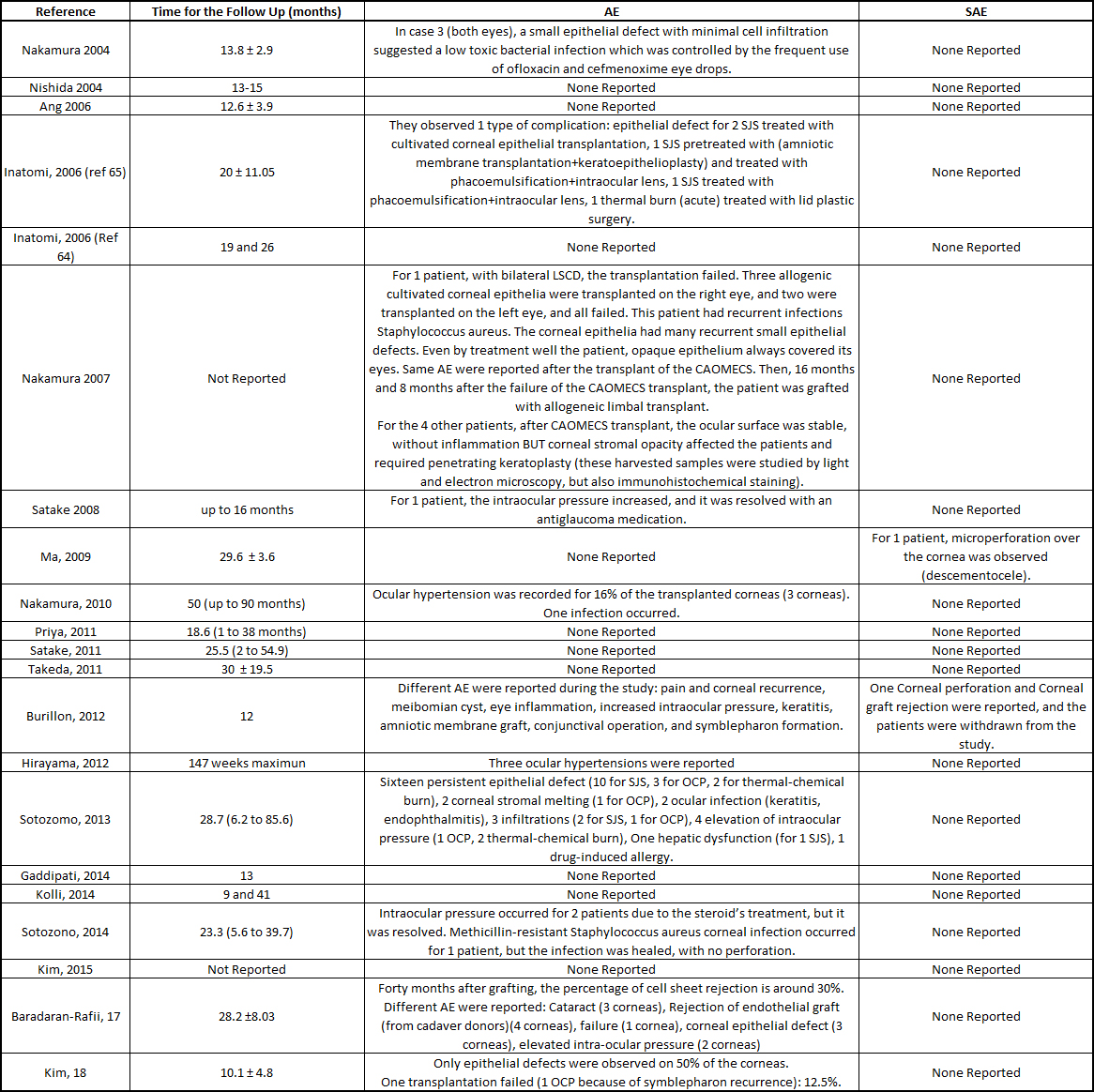
Table S3: Summarize of the patients follow up and reports of the severe and adverse events.
This entry is adapted from the peer-reviewed paper 10.3390/ijms21020411
References
- R A Thoft; J Friend; The X, Y, Z hypothesis of corneal epithelial maintenance.. Investigative Ophthalmology & Visual Science 1983, 24, 1442-1443, .
- Jinny J Yoon; Salim Ismail; Trevor Sherwin; Limbal stem cells: Central concepts of corneal epithelial homeostasis. World Journal of Stem Cells 2014, 6, 391-403, 10.4252/wjsc.v6.i4.391.
- M F Goldberg; A J Bron; Limbal palisades of Vogt.. Transactions of the American Ophthalmological Society 1982, 80, 155-171, .
- Notara, M., et al., The Role of Limbal Epithelial Stem Cells in Regulating Corneal (Lymph)angiogenic Privilege and the Micromilieu of the Limbal Niche following UV Exposure. Stem Cells Int, 2018. 2018: p. 8620172.
- Abdelfattah, N.S., et al., Clinical correlates of common corneal neovascular diseases: a literature review. Int J Ophthalmol, 2015. 8(1): p. 182-93.
- Cursiefen, C., et al., Consensus statement on indications for anti-angiogenic therapy in the management of corneal diseases associated with neovascularisation: outcome of an expert roundtable. Br J Ophthalmol, 2012. 96(1): p. 3-9.
- Azar, D.T., Corneal angiogenic privilege: angiogenic and antiangiogenic factors in corneal avascularity, vasculogenesis, and wound healing (an American Ophthalmological Society thesis). Trans Am Ophthalmol Soc, 2006. 104: p. 264-302.
- Deng, S.X., et al., Global Consensus on Definition, Classification, Diagnosis, and Staging of Limbal Stem Cell Deficiency. Cornea, 2019. 38(3): p. 364-375.
- Sacchetti, M., et al., Limbal Stem Cell Transplantation: Clinical Results, Limits, and Perspectives. Stem Cells Int, 2018. 2018: p. 8086269.
- Stacey N. Bright; M. E. Ressler; Stacey Alberts; Alberto Noriega-Crespo; Jane E. Morrison; Macarena García-Marín; Ori Fox; G. H. Rieke; Alistair C. Glasse; G. S. Wright; et al. MIRI/JWST detector characterization. Space Telescopes and Instrumentation 2016: Optical, Infrared, and Millimeter Wave 2016, 9904, 990441, 10.1117/12.2231751.
- Fernandez-Buenaga, R., et al., Twenty years of limbal epithelial therapy: an update on managing limbal stem cell deficiency. BMJ Open Ophthalmol, 2018. 3(1): p. e000164.
- R. Abdoli; S. Z. Mirhoseini; N. Ghavi Hossein-Zadeh; P. Zamani; C. Gondro; Genome-wide association study to identify genomic regions affecting prolificacy in Lori-Bakhtiari sheep. Animal Genetics 2018, 49, 488-491, 10.1111/age.12700.
- Sajjad Ahmad; Concise Review: Limbal Stem Cell Deficiency, Dysfunction, and Distress. STEM CELLS Translational Medicine 2012, 1, 110-115, 10.5966/sctm.2011-0037.
- W J Armitage; A B Tullo; D F P Larkin; The first successful full‐thickness corneal transplant: a commentary on Eduard Zirm's landmark paper of 1906. British Journal of Ophthalmology 2006, 90, 1222-1223, 10.1136/bjo.2006.101527.
- Sheraz M. Daya; Conjunctival–limbal autograft. Current Opinion in Ophthalmology 2017, 28, 370-376, 10.1097/icu.0000000000000385.
- Atallah, M.R., et al., Limbal stem cell transplantation: current perspectives. Clin Ophthalmol, 2016. 10: p. 593-602.
- Dua, H.S. and A. Azuara-Blanco, Autologous limbal transplantation in patients with unilateral corneal stem cell deficiency. Br J Ophthalmol, 2000. 84(3): p. 273-278.
- Sweder Van Wijnbergen; Tim Willems; Imperfect information, lagged labour adjustment, and the Great Moderation. Oxford Economic Papers 2012, 65, 219-239, 10.1093/oep/gps037.
- Bhalekar, S., et al., Successful autologous simple limbal epithelial transplantation (SLET) in previously failed paediatric limbal transplantation for ocular surface burns. BMJ Case Rep, 2013. 2013.
- Jayesh Vazirani; Indumathi Mariappan; Shreyas Ramamurthy; Saba Fatima; Sayan Basu; Virender S. Sangwan; Surgical Management of Bilateral Limbal Stem Cell Deficiency. The Ocular Surface 2016, 14, 350-364, 10.1016/j.jtos.2016.02.006.
- Tobias Röck; Matthias Bramkamp; Karl Ulrich Bartz-Schmidt; Daniel Röck; Organ transplantation scandal influencing corneal donation rate. International Journal of Ophthalmology 2017, 10, 1001-1003, 10.18240/ijo.2017.06.25.
- Jerry Y. Niederkorn; Immunology of Corneal Allografts: Insights from Animal Models. Journal of Clinical & Experimental Ophthalmology 2015, 6, 1-7, 10.4172/2155-9570.1000429.
- Krakauer, M., et al., Adverse effects of systemic immunosuppression in keratolimbal allograft. J Ophthalmol, 2012. 2012: p. 576712.
- Nishida, K., et al., Corneal reconstruction with tissue-engineered cell sheets composed of autologous oral mucosal epithelium. N Engl J Med, 2004. 351(12): p. 1187-1196.
- Nakamura, T., et al., Transplantation of cultivated autologous oral mucosal epithelial cells in patients with severe ocular surface disorders. Br J Ophthalmol, 2004. 88(10): p. 1280-1284.
- Kolli, S., et al., Successful application of ex vivo expanded human autologous oral mucosal epithelium for the treatment of total bilateral limbal stem cell deficiency. Stem Cells, 2014. 32(8): p. 2135-2146.
- David Hui-Kang Ma; Jan-Kan Chen; Fen Zhang; Kuei-Ying Lin; Jeng-Yuan Yao; Jau-Song Yu; Regulation of corneal angiogenesis in limbal stem cell deficiency. Progress in Retinal and Eye Research 2006, 25, 563-590, 10.1016/j.preteyeres.2006.09.001.
- Satake, Y., et al., Barrier function and cytologic features of the ocular surface epithelium after autologous cultivated oral mucosal epithelial transplantation. Arch Ophthalmol, 2008. 126(1): p. 23-28.
- Nakamura, T., et al., Long-term results of autologous cultivated oral mucosal epithelial transplantation in the scar phase of severe ocular surface disorders. Br J Ophthalmol, 2011. 95(7): p. 942-946.
- Kohji Nishida; Masayuki Yamato; Yasutaka Hayashida; Katsuhiko Watanabe; Kazuaki Yamamoto; Eijiro Adachi; Shigeru Nagai; Akihiko Kikuchi; Naoyuki Maeda; Hitoshi Watanabe; et al. Corneal Reconstruction with Tissue-Engineered Cell Sheets Composed of Autologous Oral Mucosal Epithelium. New England Journal of Medicine 2004, 351, 1187-1196, 10.1056/nejmoa040455.
- T Nakamura; T Inatomi; C Sotozono; T Amemiya; N Kanamura; S Kinoshita; Transplantation of cultivated autologous oral mucosal epithelial cells in patients with severe ocular surface disorders. Br. J. Ophthalmol. 2004, 88, 1280-1284, 10.1136/bjo.2003.038497.
- Sotozono, C., et al., Visual improvement after cultivated oral mucosal epithelial transplantation. Ophthalmology, 2013. 120(1): p. 193-200.
- Kim, Y.J., et al., Prospective Clinical Trial of Corneal Reconstruction With Biomaterial-Free Cultured Oral Mucosal Epithelial Cell Sheets. Cornea, 2018. 37(1): p. 76-83.
- Inatomi, T., et al., Ocular surface reconstruction with combination of cultivated autologous oral mucosal epithelial transplantation and penetrating keratoplasty. Am J Ophthalmol, 2006. 142(5): p. 757-764.
- Baradaran-Rafii, A., et al., Midterm outcomes of penetrating keratoplasty after cultivated oral mucosal epithelial transplantation in chemical burn. Ocul Surf, 2017. 15(4): p. 789-794.
- Priya, C.G., et al., Adult human buccal epithelial stem cells: identification, ex-vivo expansion, and transplantation for corneal surface reconstruction. Eye (Lond), 2011. 25(12): p. 1641-1649.
- Nakamura, T., et al., Phenotypic investigation of human eyes with transplanted autologous cultivated oral mucosal epithelial sheets for severe ocular surface diseases. Ophthalmology, 2007. 114(6): p. 1080-1088.
- D H-K Ma; Ming-Tse Kuo; Y-J Tsai; H-C J Chen; X-L Chen; S-F Wang; L Li; C-H Hsiao; K-K Lin; Transplantation of cultivated oral mucosal epithelial cells for severe corneal burn. Eye 2009, 23, 1442-1450, 10.1038/eye.2009.60.
- Lee R. Katzman; Bennie H. Jeng; Management strategies for persistent epithelial defects of the cornea.. Saudi Journal of Ophthalmology 2014, 28, 168-172, 10.1016/j.sjopt.2014.06.011.
- Inatomi, T., et al., Midterm results on ocular surface reconstruction using cultivated autologous oral mucosal epithelial transplantation. Am J Ophthalmol, 2006. 141(2): p. 267-275.
- Ang, L.P., et al., Autologous serum-derived cultivated oral epithelial transplants for severe ocular surface disease. Arch Ophthalmol, 2006. 124(11): p. 1543-1551.
- Kazunori Takeda; Takahiro Nakamura; Tsutomu Inatomi; Chie Sotozono; Akihide Watanabe; Shigeru Kinoshita; Ocular Surface Reconstruction Using the Combination of Autologous Cultivated Oral Mucosal Epithelial Transplantation and Eyelid Surgery for Severe Ocular Surface Disease. American Journal of Ophthalmology 2011, 152, 195-201.e1, 10.1016/j.ajo.2011.01.046.
- Burillon, C., et al., Cultured autologous oral mucosal epithelial cell sheet (CAOMECS) transplantation for the treatment of corneal limbal epithelial stem cell deficiency. Invest Ophthalmol Vis Sci, 2012. 53(3): p. 1325-1331.
- Satake, Y., et al., Long-term outcome of cultivated oral mucosal epithelial sheet transplantation in treatment of total limbal stem cell deficiency. Ophthalmology, 2011. 118(8): p. 1524-1530.
- Sotozono, C., et al., Cultivated oral mucosal epithelial transplantation for persistent epithelial defect in severe ocular surface diseases with acute inflammatory activity. Acta Ophthalmol, 2014. 92(6): p. e447-e453.
