Cellular trafficking through the endosomal–lysosomal system is essential for the transport of cargo proteins, receptors and lipids from the plasma membrane inside the cells and across membranous organelles. By acting as sorting stations, vesicle compartments direct the fate of their content for degradation, recycling to the membrane or transport to the trans-Golgi network. To effectively communicate with their neighbors, cells need to regulate their compartmentation and guide their signaling machineries to cortical membranes underlying these contact sites. Endosomal trafficking is indispensable for the polarized distribution of fate determinants, adaptors and junctional proteins. Conversely, endocytic machineries cooperate with polarity and scaffolding components to internalize receptors and target them to discrete membrane domains. Depending on the cell and tissue context, receptor endocytosis can terminate signaling responses but can also activate them within endosomes that act as signaling platforms. Therefore, cell homeostasis and responses to environmental cues rely on the dynamic cooperation of endosomal–lysosomal machineries with polarity and signaling cues.
- cell trafficking
- endocytosis
- lysosome
- signaling regulation
- polarity
- Dlg
- Scrib
- Lgl
- EGFR
- Notch
- Drosophila testis
- squamous epithelia
- intestine
- autophagy
- PAR complex
- JNK
- Rab proteins
- nephrocytes
- SOP
- Saccharomyces cerevisiae
- Arrestins
- mTOR
- TORC1
1. Introduction
2. Endocytosis in Signaling Regulation: Setting the Stage
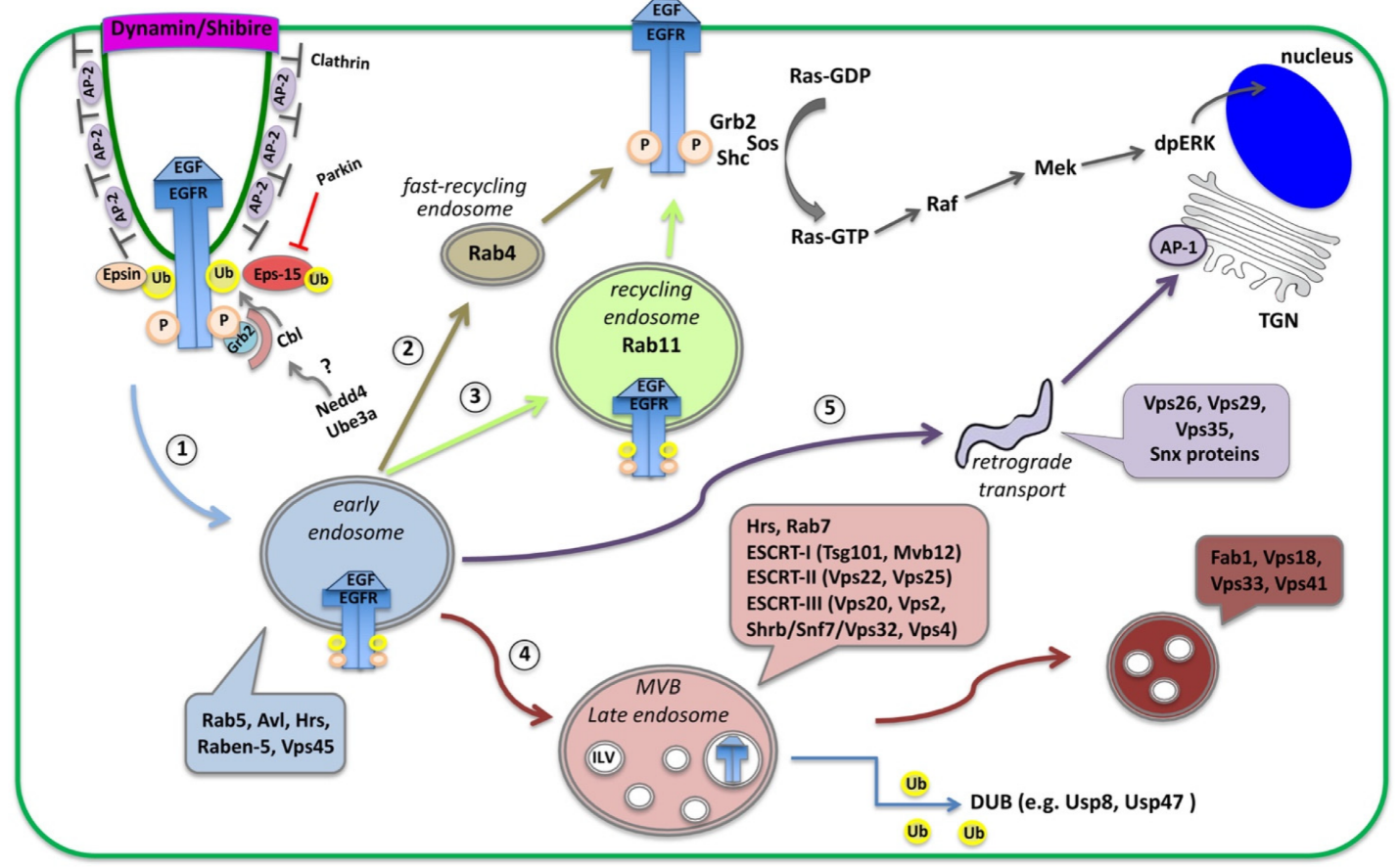
3. EGFR Signaling: Activation, Trafficking and Physiological Importance across Systems
4. Notch Signaling: Endosomal–Lysosomal Sorting and Polarization in Canonical and Non-Canonical Pathways
4.1. Endocytosis in the Canonical Model of Notch Activation
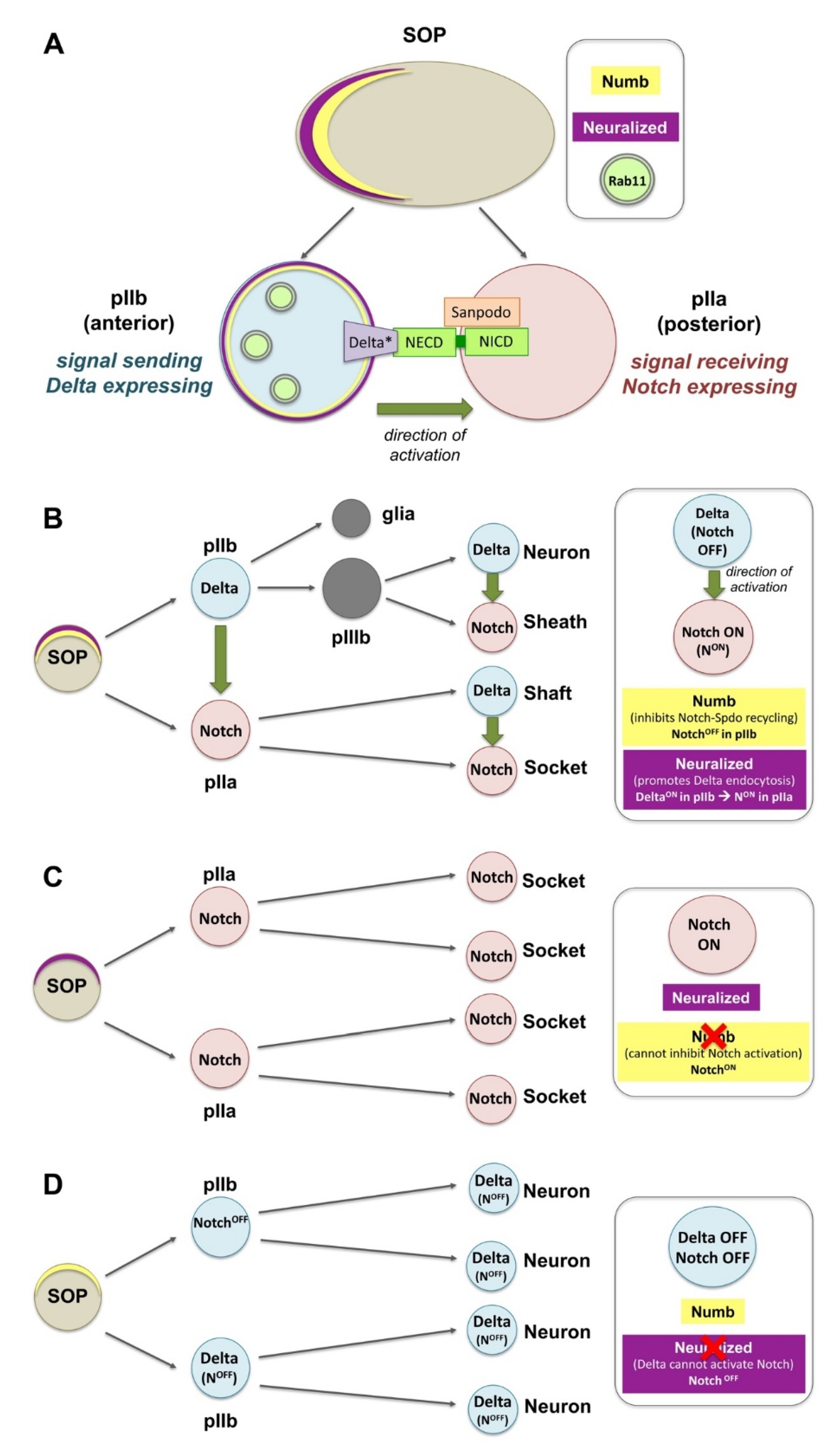
4.2. Endocytosis in the Ligand-Independent Model of Notch Activation
This entry is adapted from the peer-reviewed paper 10.3390/ijms23094684
References
- Olswang-Kutz, Y.; Gertel, Y.; Benjamin, S.; Sela, O.; Pekar, O.; Arama, E.; Steller, H.; Horowitz, M.; Segal, D. Drosophila Past1 is involved in endocytosis and is required for germline development and survival of the adult fly. J. Cell Sci. 2009, 122, 471–480.
- Kumari, S.; Mg, S.; Mayor, S. Endocytosis unplugged: Multiple ways to enter the cell. Cell Res. 2010, 20, 256–275.
- Sorkin, A.; Von Zastrow, M. Signal transduction and endocytosis: Close encounters of many kinds. Nat. Rev. Mol. Cell Biol. 2002, 3, 600–614.
- LE Roy, C.; Wrana, J.L. Clathrin- and non-clathrin-mediated endocytic regulation of cell signalling. Nat. Rev. Mol. Cell Biol. 2005, 6, 112–126.
- Mellman, I.; Nelson, W.J. Coordinated protein sorting, targeting and distribution in polarized cells. Nat. Rev. Mol. Cell Biol. 2008, 9, 833–845.
- Shivas, J.M.; Morrison, H.A.; Bilder, D.; Skop, A.R. Polarity and endocytosis: Reciprocal regulation. Trends Cell Biol. 2010, 20, 445–452.
- Zink, S.; Jacob, R. Protein trafficking in polarized epithelial cells. In Cell Polarity 1: Biological Role and Basic Mechanisms; Ebnet, K., Ed.; Springer International Publishing: Cham, Switzerland, 2015.
- Thompson, B.J. Membrane traffic and apicobasal polarity in drosophila epithelial cells. In Cell Polarity 1: Biological Role and Basic Mechanisms; Ebnet, K., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 329–348.
- Ang, S.F.; Fölsch, H. The role of secretory and endocytic pathways in the maintenance of cell polarity. Essays Biochem. 2012, 53, 29–39.
- Grossier, J.-P.; Xouri, G.; Goud, B.; Schauer, K. Cell adhesion defines the topology of endocytosis and signaling. EMBO J. 2014, 33, 35–45.
- Conte, A.; Sigismund, S. Chapter six—The ubiquitin network in the control of EGFR endocytosis and signaling. Prog. Mol. Biol. Transl. Sci. 2016, 141, 225–276.
- Dobrowolski, R.; De Robertis, E.M. Endocytic control of growth factor signalling: Multivesicular bodies as signalling organelles. Nat. Rev. Mol. Cell Biol. 2011, 13, 53–60.
- Sadowski, L.; Pilecka, I.; Miaczynska, M. Signaling from endosomes: Location makes a difference. Exp. Cell Res. 2009, 315, 1601–1609.
- Czech, M.P. PIP2 and PIP3: Complex roles at the cell surface. Cell 2000, 100, 603–606.
- Goh, L.K.; Sorkin, A. Endocytosis of receptor tyrosine kinases. Cold Spring Harb. Perspect. Biol. 2013, 5, a017459.
- Doherty, G.J.; McMahon, H.T. Mechanisms of endocytosis. Annu. Rev. Biochem. 2009, 78, 857–902.
- Renard, H.-F.; Boucrot, E. Unconventional endocytic mechanisms. Curr. Opin. Cell Biol. 2021, 71, 120–129.
- Schmid, S.L. Reciprocal regulation of signaling and endocytosis: Implications for the evolving cancer cell. J. Cell Biol. 2017, 216, 2623–2632.
- Moore, R.; Pujol, M.G.; Zhu, Z.; Smythe, E. Interplay of endocytosis and growth factor receptor signalling. Prog. Mol. Subcell. Biol. 2018, 57, 181–202.
- Chen, M.-L.; Green, D.; Liu, L.; Lam, Y.C.; Mukai, L.; Rao, S.; Ramagiri, S.; Krishnan, K.S.; Engel, J.E.; Lin, J.J.-C.; et al. Unique biochemical and behavioral alterations indrosophilashibirets1mutants imply a conformational state affecting dynamin subcellular distribution and synaptic vesicle cycling. J. Neurobiol. 2002, 53, 319–329.
- Lu, H.; Bilder, D. Endocytic control of epithelial polarity and proliferation in Drosophila. Nat. Cell Biol. 2005, 7, 1232–1239.
- Rodriguez-Boulan, E.; Kreitzer, G.; Müsch, A. Organization of vesicular trafficking in epithelia. Nat. Rev. Mol. Cell Biol. 2005, 6, 233–247.
- Wang, S.; Bellen, H.J. The retromer complex in development and disease. Development 2015, 142, 2392–2396.
- Legent, K.; Liu, H.H.; Treisman, J.E. Drosophila Vps4 promotes Epidermal growth factor receptor signaling independently of its role in receptor degradation. Development 2015, 142, 1480–1491.
- Schneider, M.; Troost, T.; Grawe, F.; Martinez-Arias, A.; Klein, T. Activation of notch in lgd mutant cells requires the fusion of late endosomes with the lysosome. J. Cell Sci. 2013, 126, 645–656.
- Sousa, L.P.; Lax, I.; Shen, H.; Ferguson, S.M.; De Camilli, P.; Schlessinger, J. Suppression of EGFR endocytosis by dynamin depletion reveals that EGFR signaling occurs primarily at the plasma membrane. Proc. Natl. Acad. Sci. USA 2012, 109, 4419–4424.
- Tomas, A.; Futter, C.E.; Eden, E.R. EGF receptor trafficking: Consequences for signaling and cancer. Trends Cell Biol. 2013, 24, 26–34.
- Jones, S.; Rappoport, J.Z. Interdependent epidermal growth factor receptor signalling and trafficking. Int. J. Biochem. Cell Biol. 2014, 51, 23–28.
- Lai, K.M.; Olivier, J.P.; Gish, G.D.; Henkemeyer, M.; McGlade, J.; Pawson, T. A Drosophila shc gene product is implicated in signaling by the DER receptor tyrosine kinase. Mol. Cell. Biol. 1995, 15, 4810–4818.
- Luschnig, S.; Krauss, J.; Bohmann, K.; Desjeux, I.; Nüsslein-Volhard, C. The Drosophila SHC adaptor protein is required for signaling by a subset of receptor tyrosine kinases. Mol. Cell 2000, 5, 231–241.
- Shilo, B.-Z. Regulating the dynamics of EGF receptor signaling in space and time. Development 2005, 132, 4017–4027.
- von Zastrow, M.; Sorkin, A. Mechanisms for regulating and organizing receptor signaling by endocytosis. Annu. Rev. Biochem. 2021, 90, 709–737.
- Krahn, M.P.; Wodarz, A. Phosphoinositide lipids and cell polarity: Linking the plasma membrane to the cytocortex. Essays Biochem. 2012, 53, 15–27.
- Caldieri, G.; Malabarba, M.G.; Di Fiore, P.P.; Sigismund, S. EGFR trafficking in physiology and cancer. In Endocytosis and Signaling; Springer: Cham, Seitzerland, 2018; Volume 57, pp. 235–272.
- Zhou, Y.; Sakurai, H. New trend in ligand-induced EGFR trafficking: A dual-mode clathrin-mediated endocytosis model. J. Proteom. 2022, 255, 104503.
- Michailidis, I.E.; Rusinova, R.; Georgakopoulos, A.; Chen, Y.; Iyengar, R.; Robakis, N.K.; Logothetis, D.E.; Baki, L. Phosphatidylinositol-4,5-bisphosphate regulates epidermal growth factor receptor activation. Pflügers Arch. Eur. J. Physiol. 2010, 461, 387–397.
- Marat, A.L.; Haucke, V. Phosphatidylinositol 3-phosphates—At the interface between cell signalling and membrane traffic. EMBO J. 2016, 35, 561–579.
- Halim, K.B.A.; Koldsø, H.; Sansom, M.S. Interactions of the EGFR juxtamembrane domain with PIP2-containing lipid bilayers: Insights from multiscale molecular dynamics simulations. Biochim. Biophys. Acta 2015, 1850, 1017–1025.
- Katzmann, D.J.; Odorizzi, G.; Emr, S.D. Receptor downregulation and multivesicular-body sorting. Nat. Rev. Mol. Cell Biol. 2002, 3, 893–905.
- Haglund, K.; Dikic, I. The role of ubiquitylation in receptor endocytosis and endosomal sorting. J. Cell Sci. 2012, 125, 265–275.
- Morreale, F.E.; Walden, H. Types of ubiquitin ligases. Cell 2016, 165, 248–248.e1.
- Husnjak, K.; Dikic, I. EGFR trafficking: Parkin’ in a jam. Nat. Cell Biol. 2006, 8, 787–788.
- Pai, L.-M.; Wang, P.-Y.; Chen, S.-R.; Barcelo, G.; Chang, W.-L.; Nilson, L.; Schüpbach, T. Differential effects of Cbl isoforms on Egfr signaling in drosophila. Mech. Dev. 2006, 123, 450–462.
- Wang, Y.; Chen, Z.; Bergmann, A. Regulation of EGFR and notch signaling by distinct isoforms of D-cbl during drosophila development. Dev. Biol. 2010, 342, 1–10.
- Henegouwen, P.M.V.B.E. Eps15: A multifunctional adaptor protein regulating intracellular trafficking. Cell Commun. Signal. 2009, 7, 24.
- Weber, J.; Polo, S.; Maspero, E. HECT E3 Ligases: A tale with multiple facets. Front. Physiol. 2019, 10, 370.
- Fallon, L.; Bélanger, C.M.; Corera, A.T.; Kontogiannea, M.; Regan-Klapisz, E.; Moreau, F.; Voortman, J.; Haber, M.; Rouleau, G.; Thorarinsdottir, T.; et al. A regulated interaction with the UIM protein Eps15 implicates parkin in EGF receptor trafficking and PI(3)K—Akt signalling. Nat. Cell Biol. 2006, 8, 834–842.
- Lobert, V.H.; Stenmark, H.; Divakaruni, A.S.; Brand, M.D. Cell polarity and migration: Emerging role for the endosomal sorting machinery. Physiology 2011, 26, 171–180.
- Sorkin, A.; von Zastrow, M. Endocytosis and signalling: Intertwining molecular networks. Nat. Rev. Mol. Cell Biol. 2009, 10, 609–622.
- Vaccari, T.; Bilder, D. At the crossroads of polarity, proliferation and apoptosis: The use of Drosophila to unravel the multifaceted role of endocytosis in tumor suppression. Mol. Oncol. 2009, 3, 354–365.
- Bache, K.G.; Stuffers, S.; Malerød, L.; Slagsvold, T.; Raiborg, C.; Lechardeur, D.; Wälchli, S.; Lukacs, G.L.; Brech, A.; Stenmark, H. The ESCRT-III subunit hVps24 is required for degradation but not silencing of the epidermal growth factor receptor. Mol. Biol. Cell 2006, 17, 2513–2523.
- Babst, M.; Katzmann, D.J.; Estepa-Sabal, E.J.; Meerloo, T.; Emr, S.D. Escrt-III: An endosome-associated heterooligomeric protein complex required for mvb sorting. Dev. Cell 2002, 3, 271–282.
- Vaccari, T.; Rusten, T.E.; Menut, L.; Nezis, I.P.; Brech, A.; Stenmark, H.; Bilder, D. Comparative analysis of ESCRT-I, ESCRT-II and ESCRT-III function in Drosophila by efficient isolation of ESCRT mutants. J. Cell Sci. 2009, 122, 2413–2423.
- Sheng, Z.; Yu, L.; Zhang, T.; Pei, X.; Li, X.; Zhang, Z.; Du, W. ESCRT-0 complex modulates Rbf mutant cell survival by regulating Rhomboid endosomal trafficking and EGFR signaling. J. Cell Sci. 2016, 129, 2075–2084.
- Olivares-Castiñeira, I.; Llimargas, M. EGFR controls Drosophila tracheal tube elongation by intracellular trafficking regulation. PLoS Genet. 2017, 13, e1006882.
- Castanieto, A.; Johnston, M.J.; Nystul, T.G. EGFR signaling promotes self-renewal through the establishment of cell polarity in Drosophila follicle stem cells. eLife 2014, 3, e04437.
- Kuwada, S.K.; Lund, K.A.; Li, X.F.; Cliften, P.; Amsler, K.; Opresko, L.K.; Wiley, H.S. Differential signaling and regulation of apical vs. basolateral EGFR in polarized epithelial cells. Am. J. Physiol. Content 1998, 275, C1419–C1428.
- Amsler, K.; Kuwada, S.K. Membrane receptor location defines receptor interaction with signaling proteins in a polarized epithelium. Am. J. Physiol. Content 1999, 276, C91–C101.
- Singh, B.; Coffey, R.J. Trafficking of epidermal growth factor receptor ligands in polarized epithelial cells. Annu. Rev. Physiol. 2014, 76, 275–300.
- Shang, P.; Stepicheva, N.; Teel, K.; McCauley, A.; Fitting, C.S.; Hose, S.; Grebe, R.; Yazdankhah, M.; Ghosh, S.; Liu, H.; et al. βA3/A1-crystallin regulates apical polarity and EGFR endocytosis in retinal pigmented epithelial cells. Commun. Biol. 2021, 4, 850.
- Kim, S.; Nahm, M.; Kim, N.; Kwon, Y.; Kim, J.; Choi, S.; Choi, E.Y.; Shim, J.; Lee, C.; Lee, S. Graf regulates hematopoiesis through GEEC endocytosis of EGFR. Development 2017, 144, 4159–4172.
- Henriksen, L.; Grandal, M.V.; Knudsen, S.L.J.; van Deurs, B.; Grøvdal, L.M. Internalization mechanisms of the epidermal growth factor receptor after activation with different ligands. PLoS ONE 2013, 8, e58148.
- Vermeer, P.D.; Einwalter, L.A.; Moninger, T.O.; Rokhlina, T.; Kern, J.; Zabner, J.; Welsh, M.J. Segregation of receptor and ligand regulates activation of epithelial growth factor receptor. Nature 2003, 422, 322–326.
- Fürthauer, M.; González-Gaitán, M. Endocytic regulation of notch signalling during development. Traffic 2009, 10, 792–802.
- Yamamoto, S.; Charng, W.-L.; Bellen, H.J. Endocytosis and intracellular trafficking of notch and its ligands. Curr. Top. Dev. Biol. 2010, 92, 165–200.
- Daeden, A.; Gonzalez-Gaitan, M. Endosomal trafficking during mitosis and notch-dependent asymmetric division. Prog. Mol. Subcell. Biol. 2018, 57, 301–329.
- Keder, A.; Carmena, A. Cytoplasmic protein motility and polarized sorting during asymmetric cell division. Wiley Interdiscip. Rev. Dev. Biol. 2013, 2, 797–808.
- Tien, A.-C.; Rajan, A.; Bellen, H.J. A Notch updated. J. Cell Biol. 2009, 184, 621–629.
- Fiuza, U.-M.; Arias, A.M. Cell and molecular biology of Notch. J. Endocrinol. 2007, 194, 459–474.
- Hounjet, J.; Vooijs, M. The role of intracellular trafficking of notch receptors in ligand-independent notch activation. Biomolecules 2021, 11, 1369.
- Revici, R.; Hosseini-Alghaderi, S.; Haslam, F.; Whiteford, R.; Baron, M. E3 Ubiquitin ligase regulators of notch receptor endocytosis: From flies to humans. Biomolecules 2022, 12, 224.
- Tomlinson, A.; Struhl, G. Delta/notch and boss/sevenless signals act combinatorially to specify the drosophila R7 photoreceptor. Mol. Cell 2001, 7, 487–495.
- Polesello, C.; Tapon, N. Salvador-warts-hippo signaling promotes drosophila posterior follicle cell maturation downstream of notch. Curr. Biol. 2007, 17, 1864–1870.
- Chen, H.-J.; Wang, C.-M.; Wang, T.-W.; Liaw, G.-J.; Hsu, T.-H.; Lin, T.-H.; Yu, J.-Y. The hippo pathway controls polar cell fate through notch signaling during drosophila oogenesis. Dev. Biol. 2011, 357, 370–379.
- Huang, H.; Kornberg, T.B. Myoblast cytonemes mediate Wg signaling from the wing imaginal disc and delta-notch signaling to the air sac primordium. eLife 2015, 4, e06114.
- Doroquez, D.B.; Rebay, I. Signal integration during development: Mechanisms of EGFR and notch pathway function and cross-talk. Crit. Rev. Biochem. Mol. Biol. 2006, 41, 339–385.
- Nagaraj, R.; Banerjee, U. Regulation of notch and wingless signalling by phyllopod, a transcriptional target of the EGFR pathway. EMBO J. 2009, 28, 337–346.
- Kitadate, Y.; Kobayashi, S. Notch and Egfr signaling act antagonistically to regulate germ-line stem cell niche formation in Drosophila male embryonic gonads. Proc. Natl. Acad. Sci. USA 2010, 107, 14241–14246.
- Reiff, T.; Antonello, Z.A.; Ballesta-Illán, E.; Mira, L.; Sala, S.; Navarro, M.; Martinez, L.M.; Dominguez, M. Notch and EGFR regulate apoptosis in progenitor cells to ensure gut homeostasis in Drosophila. EMBO J. 2019, 38, e101346.
- Blackie, L.; Tozluoglu, M.; Trylinski, M.; Walther, R.F.; Schweisguth, F.; Mao, Y.; Pichaud, F. A combination of Notch signaling, preferential adhesion and endocytosis induces a slow mode of cell intercalation in the Drosophila retina. Development 2021, 148, dev197301.
- Hori, K.; Sen, A.; Kirchhausen, T.; Artavanis-Tsakonas, S. Synergy between the ESCRT-III complex and Deltex defines a ligand-independent Notch signal. J. Cell Biol. 2011, 195, 1005–1015.
- Shimizu, H.; Wilkin, M.B.; Woodcock, S.A.; Bonfini, A.; Hung, Y.; Mazaleyrat, S.; Baron, M. The Drosophila ZO-1 protein Polychaetoid suppresses Deltex-regulated Notch activity to modulate germline stem cell niche formation. Open Biol. 2017, 7, 160322.
- Bellec, K.; Pinot, M.; Gicquel, I.; Le Borgne, R. Clathrin adaptor AP-1 and Stratum act in parallel pathways to control Notch activation in drosophila sensory organ precursor cells. Development 2021, 148, dev191437.
- Couturier, L.; Mazouni, K.; Schweisguth, F. Inhibition of notch recycling by numb: Relevance and mechanism(s). Cell Cycle 2013, 12, 1647–1648.
- Johnson, S.A.; Zitserman, D.; Roegiers, F. Numb regulates the balance between Notch recycling and late-endosome targeting in Drosophilaneural progenitor cells. Mol. Biol. Cell 2016, 27, 2857–2866.
- Cotton, M.; Benhra, N.; Le Borgne, R. Numb inhibits the recycling of sanpodo in drosophila sensory organ precursor. Curr. Biol. 2013, 23, 581–587.
- Medina-Yáñez, I.; Olivares, G.H.; Vega-Macaya, F.; Mlodzik, M.; Olguín, P. Phosphatidic acid increases Notch signalling by affecting Sanpodo trafficking during Drosophila sensory organ development. Sci. Rep. 2020, 10, 21731.
- Trylinski, M.; Schweisguth, F. Activation of Arp2/3 by WASp is essential for the endocytosis of delta only during cytokinesis in drosophila. Cell Rep. 2019, 28, 1–10.e3.
- Sigismund, S.; Confalonieri, S.; Ciliberto, A.; Polo, S.; Scita, G.; Di Fiore, P.P. Endocytosis and signaling: Cell logistics shape the eukaryotic cell plan. Physiol. Rev. 2012, 92, 273–366.
- Vaccari, T.; Lu, H.; Kanwar, R.; Fortini, M.E.; Bilder, D. Endosomal entry regulates Notch receptor activation in Drosophila melanogaster. J. Cell Biol. 2008, 180, 755–762.
