Endocytosis and vesicle trafficking play an equally important role in establishing polarity by coordinating intracellular protein trafficking and the distribution of protein-sorting signals to distinct plasma membrane domains [
5,
6,
7]. In some cell types, such as
Drosophila neuroblasts, polarity relies more on the differential distribution of cell fate determinants, while classical epithelia polarity involves the differential localization of transmembrane and adaptor proteins or targeting of the transport machinery across apical vs. basolateral membranes [
5,
8,
9] (Figure 3C). Reciprocally, polarized cytoskeletal, adaptor and scaffolding proteins participate in binding and directing endosomal, membrane trafficking components and signaling platforms or exocytic delivery to specific subcellular domains. For example, restriction of endocytosis to micropatterns, defined by cell adhesion geometry, can define the topology of signaling reception and downstream propagation [
10]. Although endocytosis cooperates with cytoskeletal and adhesion proteins, to direct the polarized distribution of receptor-containing vesicles inside the cells, in many cases, localized signaling reception can also define the subcellular delivery of endocytic and cargo-sorting events [
5]. Therefore, the interplay of endocytosis with polarity and signaling seems to be highly dynamic and context-dependent.
2. Endocytosis in Signaling Regulation: Setting the Stage
Signaling is critically required for cell-coordinated function and for setting up complex tissues. Cells communicate with each other through the activation of receptors located at their surface plasma membrane. However, the fine trimming of signaling levels is also critical for proper cellular output and maintaining homeostasis. Signal attenuation is based on the internalization and removal of activated ligand-receptor complexes from the membrane via endocytosis [
13,
14,
15]. Endocytosis of activated receptors follows clathrin-mediated endocytosis (CME) [
13,
16,
17] but can also follow clathrin-independent pathways, e.g., caveolar-type (reviewed in [
18,
19,
20,
21]). In CME, activated receptors are recruited to clathrin-coated pits by interacting with the adaptor protein (AP)-2 complex, then clathrin-coated pits invaginate and pinch off with the action of the GTPase Dynamin, encoded by the
shibire gene in
Drosophila [
13,
17,
22] (
Figure 1). Another constituent of clathrin-coated membranes is the membrane phospholipid Phosphatidylinositol 4,5-bisphosphate [PtdIns(4,5)P2] (PIP2), that binds and recruits AP-2 [
16].
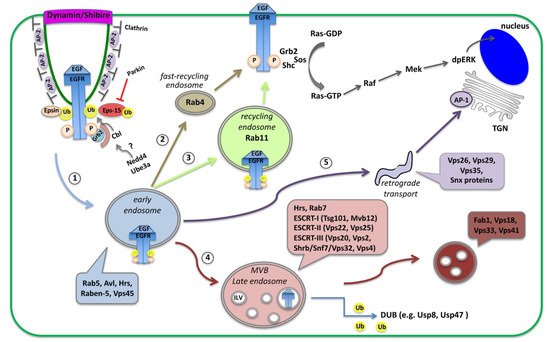
Figure 1. Schematic diagram of receptor endocytosis and trafficking routes within the cells. Here, the EGFR is shown as an example of receptor endocytosis, along with adaptor proteins recruited upon binding of the EGF ligand. Activation of the EGFR initiates the Ras/MAPK cascade that leads to double phosphorylation of the MAPK (dpERK), which translocates into the nucleus and activates transcription. Activated EGFR is removed from the plasma membrane via clathrin-mediated endocytosis (CME). Receptors loaded on Rab5-containing early endosomes (1), can follow alternative routes and (2) recycle back to the membrane by a Rab4 fast recycling endosome, (3) traffic to Rab11-containing recycling endosome, (4) proceed to multi-vesicular bodies (MVB), late endosomes and lysosomes for degradation, or (5) traffic to sorting endosomes or to the trans-Golgi network (TGN) via retromer trafficking.
Once loaded on endosomes, internalized receptors can be either recycled back to the cell surface or transported to lysosomes for degradation. Initially, receptor-containing vesicles fuse to the early endosome (EE) where endosomal trafficking is controlled by the Rab proteins, small GTP-binding proteins of the Ras superfamily. Each Rab protein resides in a particular type of endosome and recruits specific effector proteins. EEs, containing Rab5 and the syntaxin7 Avalance (Avl) [
23], can follow several alternative routes: (1) rapidly recycle back to the membrane by a Rab4-dependent mechanism; (2) traffic to the Rab11-containing recycling endosome (RE) to recycle to the plasma membrane; (3) proceed to multi-vesicular bodies (MVB), late endosomes (LE) and lysosomes for degradation; or (4) traffic to the
trans-Golgi network (TGN) in the so-called retromer trafficking or to sorting endosomes that mediate apical or basolateral trafficking [
24,
25] (
Figure 1). MVBs are defined by the presence of intra-luminal vesicles (ILV) that are formed in a process of inward membrane invagination involving the Endosomal Sorting Complex Required for Transport (ESCRT) complexes. The formation of ILV within MVBs is a critical step in signaling regulation. Before delivery into the ILVs, receptors can still bind their ligands and continue signaling through their cytoplasmic domain. Internalization of receptors into the ILVs segregates their intracellular domain from the cytoplasm and terminates signaling [
26,
27]. Maturation of EE to LE involves the acquisition of Rab7 and loss of Rab proteins that are involved in receptor recycling (
Figure 1). Interestingly, in many cases receptor internalization is important not for silencing the receptor but to transport the ligand-receptor complexes into the cell for signal maintenance or propagation in endosomal compartments that act as signaling platforms [
15,
28,
29].
3. EGFR Signaling: Activation, Trafficking and Physiological Importance across Systems
Epidermal Growth Factor Receptor (EGFR) is a receptor tyrosine kinase (RTK), which upon ligand binding, it dimerizes, becomes phosphorylated and a complex of signaling molecules assembles and initiates the downstream MAPK/Ras signaling cascade [
14,
30]. EGFR phosphorylation leads to the recruitment of signal-transducing adaptor proteins Grb2, the
Drosophila Downstream of Receptor Kinase (Drk), and SHC-adaptor protein (Shc) [
17,
31,
32], which allow the assembly of signaling complexes on the cytoplasmic tail of the activated EGFR. Grb2 recruits Sos (Son of Sevenless) but Shc is also able to link the Grb2-Sos complex to the activated EGFR. Sos stimulates Ras activation and initiates the MAPK cascade with a series of activation events that lead to double phosphorylation of the MAPK dpERK, which translocates to the nucleus and activates transcription [
21,
30,
33] (
Figure 1).
At the same time, EGFR phosphorylation activates the recruitment of the adaptor proteins that initiate the endocytic removal from the membrane. This process is seen in several tissues and organs across species, and deregulation of this process is implicated in cancer initiation and progression [
13,
14]. EGFR becomes endocytosed mainly by clathrin-mediated endocytosis (CME), which happens at both low and high EGFR physiological doses [
12,
13,
16,
17,
34,
35]. High-saturated doses of EGF ligands induce in parallel clathrin-independent endocytosis (CIE) of the EGFR [
13,
21,
35,
36]. The membrane phospholipid PtdIns(4,5)P2 (PIP2) could potentially link endocytosis to the EGFR signaling as it not only recruits AP-2 to clathrin-coated pits, but also physically binds the EGFR juxtamembrane domain and enhances EGFR phosphorylation and activation in many tissues [
37,
38,
39]. Moreover, the adaptor proteins Epsin and Eps15 (epidermal growth factor substrate 15) link the activated EGFR to the clathrin coat by binding to Clathrin and AP-2. Once inside the cell, EGFR-containing vesicles can recycle back to the cell surface or get transported to lysosomes for degradation or even to other parts of the cells via sorting endosomes [
12,
24,
35].
Besides phosphorylation, ubiquitination is another critical post-translational modification for EGFR endocytosis, receptor trafficking and signaling downregulation. Ubiquitin is a highly conserved 76 amino acid polypeptide that becomes covalently linked to protein substrates including receptors [
40]. The ubiquitin-activating enzyme (E1), ubiquitin-conjugating enzyme (E2) and ubiquitin ligase (E3) drive ubiquitination through the addition of a small ubiquitin (Ub) protein to the EGFR (EGFR-Ub). Mono-ubiquitination of receptor Lysine (Lys) residues, affects receptor internalization and multivesicular-body (MVB) sorting, while poly-ubiquitination (also on Lys residues of the Ubiquitin) directs proteins to degradation by the proteasome [
40,
41]. Thus, ubiquitin modification typically downregulates the targeted receptors and protein substrates. In
Drosophila, Cbl (the homologue for Casitas B-lineage Lymphoma proto-oncogene) is the major E3 ligase and a negative regulator of EGFR signaling. E3 ligases of the Cbl family are major regulators of the EGFR pathway, acting both as ubiquitin ligases and multiadaptor molecules [
13]. Cbl E3 ligases, are RING finger containing ubiquitin ligases that mediate a direct transfer of ubiquitin to the substrate, functioning as a scaffold to orient the ubiquitin-charged E2 with respect to the substrate protein [
13,
42]. Once recruited to active EGFRs, Cbl gets phosphorylated and ubiquitinates the EGFR [
13,
17,
29,
43]. Alternative splicing of Cbl in
Drosophila creates (1) a long CblL form, specific to EGFR that binds the
Drosophila Drk, and (2) a shorter CblS form that regulates EGFR but primarily acts on Notch endocytosis [
33,
44,
45]. Therefore, Cbl and Eps-15 are both loaded onto the internalized EGFR endosomes.
Besides the ubiquitination of the receptor, the CME of the EGFR is also regulated by ubiquitination of its adaptors proteins, further complicating the picture [
13]. Epsin and Eps-15 contain ubiquitin interacting motifs (UIMs) that allow them to bind the ubiquitinated forms of EGFR but also become ubiquitinated by E3 ligases. Eps-15 ubiquitination is performed by the Nedd4 family and Parkin E3 ligases via distinct mechanisms [
46]. Once Nedd4 is self-ubiquitinated it can bind the Eps-15 UIM2 domain and ubiquitinate Eps-15. Nedd4 ligases contain tryptophan-tryptophan (WW) motifs and a HECT (homologous to the E6AP carboxyl terminus) domain that catalyzes ubiquitin addition through a two-step reaction: ubiquitin is first transferred to a catalytic cysteine on the E3 and then from the E3 to a Lys on the target substrate [
13,
42,
47]. Besides Eps-15, Nedd4 E3 ligases are also involved in EGFR endocytosis by ubiquitinating the EGFR or Cbl [
13,
17,
43]. Parkin is an E3-ubiquitin ligase of the RBR (RING-betweenRING–RING) family that catalyzes ubiquitin transfer through a two-step reaction (ubiquitin is first transferred to a catalytic cysteine on the E3 and then to the substrate) [
42,
47]. Parkin contains a ubiquitin-like (UBL) domain, which binds to the UIMs of Eps15 [
46]. Ubiquitinated Eps-15 can no longer bind the EGFR and promote its endocytosis and the EGFR signaling remains active [
43,
48]. Thus, Parkin negatively regulates EGFR endocytosis by ubiquitinating Eps-15 [
43,
48]. Taken together, E3 ligases negatively regulate the substrate proteins they target, but how this affects the EGFR signaling is context-dependent.
In the endosomes, ubiquitin serves as a molecular signature for recognition by numerous endocytic adaptor proteins and for trafficking the EGFR cargo to MVBs and lysosomes for degradation. EGFR-Ub present on REs moves to MVBs and is recognized by the ESCRT-0 component Hepatocyte growth factor regulated tyrosine kinase substrate (Hrs), which can also bind Ub, and facilitate the recruitment of ESCRT-I, ESCRT-II and ESCRT-III onto the MVB membranes (
Figure 1) [
49]. EGFR-Ub is finally internalized in ILVs inside the MVBs via the action of ESCRT-III, which promotes the inward membrane invagination of MVBs. Interestingly, EGFR molecules impaired for ubiquitination cannot be degraded, are poorly incorporated into MVBs and are recycled back to the plasma membrane [
12,
13,
29]. Therefore, EGFR-Ub needs to be maintained until ILV formation. Nevertheless, before entry into ILVs, EGFR needs to be de-ubiquitinated with the help of de-ubiquitinating enzymes (DUBs), which regulate the recycling of ubiquitin, as well as the Hrs and Eps-15 turnover. The available literature on EGFR agrees that EGFR-Ub in endosomal compartments is fully functional and can still signal until the ubiquitin modification is removed by DUBs. The precise timing of de-ubiquitination and whether it happens before or after the ubiquitin-dependent EGFR-ESCRT interaction remains to be elucidated [
17]. Results from different tissues and organisms have led to variable results and the possibility of a tissue-specific timing cannot be excluded.
Along this line of evidence, loss of ESCRT components in some tissues results in increased EGFR signaling while in others in EGFR downregulation [
11,
14,
17,
50]. In yeast and human HeLa cells, the loss of ESCRT-I and -II sustains EGFR signaling but the loss of ESCRT-III components does not [
14,
51,
52]. In
Drosophila eye discs, the loss of ESCRT-I, -II or -III components leads to increased EGFR signaling, probably from endocytic vesicles [
50,
53]. Interestingly in imaginal discs, the Vacuolar protein sorting 4 (Vps4) promotes Epidermal growth factor receptor signaling independently of its canonical role in receptor degradation (and ILV formation) as part of the ESCRT-III complex. Vps4 performs this function by acting at the level of the receptor through an endocytosis-independent mechanism and in a tissue-specific way, since, in ovarian follicles, Vps4 is involved only in EGFR degradation [
26].
On the other hand, the loss of ESCRT-0 components, such as Hrs, leads to the recycling of the EGFR receptor, which is not trapped in MVBs [
11,
17]. In developing imaginal discs, the ESCRT-0 components Hrs and Signal transducing adaptor molecule (Stam) play a role in silencing the EGFR by affecting the secreted form of Spitz (sSpi) [
54]. EGFR signaling activation is mediated by the membrane-tethered ligand Spitz (mSpi), which requires processing by the membrane protease Rhomboid to form the sSpi. Besides its effect on Spi, Rhomboid can also cleave Star, which also mediates the post-transcriptional processing of Spi [
33]. Mutations in
stam and
hrs cause the accumulation of Rhomboid in abnormal endosomal compartments and silence the EGFR before ligand binding [
54].
Several pieces of evidence show that the EGFR-containing endosomal compartments can follow different or alternative trafficking routes depending on the specific cell type, the concentration of the ligand, the developmental stage and the cellular conditions [
11,
17]. In
Drosophila trachea, EGFR signaling levels control the length of the tracheal tubes, by regulating the organization of endosomes in which Crumbs and Serpent proteins are loaded. EGFR loaded on those endosomes acts as a critical hub for the correct delivery of Crumbs (mediating apical membrane growth) and Serpent (modifier of the apical extra-cellular matrix) to their final destinations [
55]. Moreover, EGFR is involved in polarity establishment by activating cell polarity regulators beyond the canonical Ras/MAPK pathway, such as the liver kinase LKB1 in
Drosophila follicle stem cells [
56]. The polarized distribution of EGFR is also crucial since, in many polarized epithelial cells, EGFR is localized primarily at basolateral sites [
57,
58,
59]. Analysis of the retinal pigmented epithelium (RPE) in mice, revealed the critical role of the βA3/A1-crystallin in CME of the EGFR and the organization of the actin apical network [
60]. βA3/A1-crystallin maintains the PIP2 pool in the RPE by attenuating the PLCγ signaling. This activates Ezrin phosphorylation and promotes EGFR internalization, which affects RPE cell polarity [
60]. Another mechanism uncovered in plasmatocytes (the
Drosophila macrophage-like hemocytes), revealed differential endocytosis of the EGFR based on ligand levels driving EGFR receptor activation [
61]. More precisely, at high (but not low) levels of the ligand Spi, the EGFR is internalized in a clathrin-independent way via Graf (GTPase regulator associated with focal adhesion kinase), which is part of the GPI-enriched endocytic compartment (GEEC) endocytosis pathway. Graf interacts with the EGFR in a ubiquitination-dependent manner and promotes EGFR degradation and signaling attenuation [
61].
In HeLa cells, analysis of EGFR internalization following activation with different ligands has shown that EGFR endocytosis after treatment with all ligands could be inhibited to a certain degree by ablation of clathrin, which confirms the existence of an alternative CIE pathway [
62]. However, knockdown of clathrin could fully inhibit EGFR degradation with all ligands tested. The inhibition of dynamin function blocked EGFR internalization after stimulation by any of the ligands, suggesting a dynamin involvement in both CME and CIE pathways. Finally, knocking down a number of clathrin-independent dynamin-dependent pathways of internalization had no effect on the ligand-induced endocytosis of the EGFR [
62].
Taken together, EGFR endocytosis and subsequent trafficking through endocytic organelles, form a dynamic network of subcellular compartments, which actively control the timing, amplitude and specificity of the signaling. Furthermore, the distribution of EGFR in apical and/or basolateral cell surfaces can have important biological consequences that affect EGFR downregulation efficiency and endocytic turnover, and influence paracrine vs. autocrine activation by different EGF ligands [
35,
57,
58,
59,
63]. It would be interesting to understand how adaptor proteins and signaling complexes, often called “signalosomes”, find their subcellular way and how cortical and scaffolding proteins regulate the accessibility or subcellular trafficking of these proteins. Although a great number of elegant biochemical studies have identified binding partners and adaptor proteins involved in these processes, a lot of questions on the critical switch between EGFR recycling vs. degradation, the decisive timing of EGFR phosphorylation and ubiquitination, and its physiological importance in a cell-type-specific way, remain open.
4. Notch Signaling: Endosomal–Lysosomal Sorting and Polarization in Canonical and Non-Canonical Pathways
The cooperative action of endocytosis and polarity in signaling regulation is very well-illustrated in the case of Notch signaling. Research over the past decades not only revealed the regulatory patterns and alternative pathways of Notch regulation but shed light on the diversity of these networks in specific cell and tissue contexts, and across organisms (reviewed in [
64,
65,
66,
67,
68,
69,
70,
71]). Notch is a single-pass transmembrane receptor involved in cell fate decisions, morphogenetic changes (such as cell intercalation) and crosstalk with other signaling pathways [
45,
72,
73,
74,
75,
76,
77,
78,
79,
80].
4.1. Endocytosis in the Canonical Model of Notch Activation
In the canonical model, Notch binds one of the membrane-bound ligands Delta (Dl), Serrate (Ser) or Lag2 (the DSL family) from the neighboring signaling cell. This interaction initiates a cascade of proteolytic cleavages by γ-secretase that releases the intra-cellular domain of Notch (NICD). NICD can now translocate to the nucleus and activate transcription together with the transcription factor Suppressor of Hairless (Su(H)) and the nuclear effector Mastermind [
81,
82,
83]. Although several factors that regulate Notch signaling attenuation and lysosomal degradation have been identified, internalization and endosomal sorting of Notch-NICD typically leads to activation of the receptor [
50,
81].
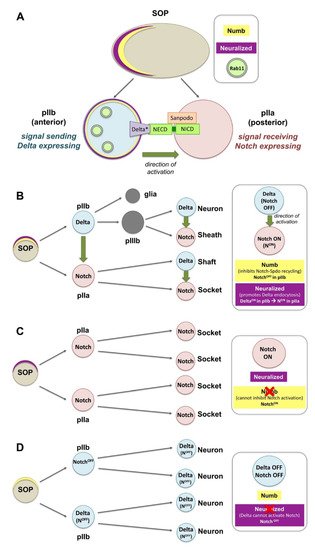
Canonical Notch activation via Delta is involved in the asymmetric cell division of the sensory organ precursors (SOPs) in the pupal notum of
Drosophila. SOPs are polarized epithelial cells that divide asymmetrically to give rise to two daughter cells, the posterior pIIa and anterior pIIb, which in turn divide asymmetrically to generate the four cells that will form the sensory organs: a neuron, a sheath, a shaft, and a socket [
64,
65,
66,
67] (
Figure 2A,B). The differential activation of Notch relies on the asymmetric distribution of the cell fate determinants Neuralized (Neur) and Numb in the anterior side of the SOP and upon division, they get segregated into the pIIb (
Figure 2A,B). Neur promotes the ubiquitination and endocytosis of Delta and thereby its activation (Delta*;
Figure 2A). Numb inhibits the recycling of Notch and its transmembrane co-factor Sanpodo (Spdo) towards the plasma membrane (promoting their degradation). Activated Delta through Rab11-positive endosomes recycles back to the apical cell surface to activate Notch in the pIIa cell. Thus, pIIb turns off Notch, while the pIIa cell (in the absence of Neur and Numb) activates Notch and Spdo facilitates the reception of the signal. Loss of
numb can no longer inhibit Notch-Spdo recycling in the anterior pIIb, Notch gets activated in the anterior precursor cell which adapts the pIIa fate, and cells finally adapt the socket fate (
Figure 2C). Conversely, loss of
neur can no longer activate Delta in pIIb, and Notch cannot be activated in pIIa, which now adapts the pIIb fate, and all cells eventually become neurons (
Figure 2D) [
64,
65,
66,
67].
Figure 2. Notch activation and asymmetric distribution of fate determinants in Drosophila sensory organ precursors (SOPs). (A) The differential activation of Notch relies on the asymmetric distribution of the cell fate determinants Neuralized (Neur) and Numb in the anterior side of the SOP. Upon division, Neur and Numb get segregated into the pIIb. Numb inhibits the recycling of Notch and Sanpodo (Spdo) directing them to degradation. Neur promotes the ubiquitination and endocytosis of Delta, and thereby its activation (Delta*). Delta* recycles to the membrane via Rab11 recycling endosomes, so that it can bind the extracellular domain of Notch (the internalization events for Notch, Spdo and Delta are not shown in this diagram). (B) SOPs are polarized epithelial cells that divide asymmetrically to give rise to a posterior pIIa and anterior pIIb. The latter divide further to generate a neuron, a sheath, a shaft, and a socket. (C) Loss of numb can no longer inhibit Notch-Spdo recycling in the anterior pIIb where Notch now stays active, and all cells adapt the socket fate. (D) Loss of neur can no longer activate Delta in pIIb, Notch is not activated in the pIIa and all cells give rise to neurons.
The regulated trafficking of Notch and Spdo plays an important role in this process. Numb interacts with internalized Spdo-Notch oligomers at sorting pIIb endosomes and inhibits the recycling of Notch, thereby creating an asymmetry in Notch distribution along the pIIa–pIIb interface [
84]. Numb also controls the Notch receptor targeting the Rab7-containing late endosomes, a specific subpopulation of Notch endosomes. In
numb mutants, the increased numbers of Notch signaling endosomes recycle to the membrane in the Rab11-dependent way [
85]. Basolateral localization of Notch in the SOP is also regulated by AP-1 and the chaperone Stratum that follows two parallel transport routes [
83]. Loss of their function leads to Notch enrichment at the apical side of the pIIa–pIIb interface. Moreover, Numb interacts with AP-1 to regulate the basolateral recycling of Spdo, while loss of
numb permits Spdo internalization and recycling back to the plasma membrane [
86]. On the other hand, phosphatidic acid (derived from Phospholipase D) promotes ectopic Notch signaling by increasing Notch endocytosis and inhibiting Sanpodo trafficking towards acidic endosomes [
87].
The Actin-related protein (Arp) 2/3 complex and its activators, Scar/WAVE and Wiskott–Aldrich Syndrome protein (WASp), promote actin polymerization and influence cell shape and motility. During SOP cytokinesis, Arp2/3 and WASp are required for the recycling of Delta [
88]. On the other hand, the Arp2/3 activator SCAR regulates contact expansion between pIIa and pIIb. Efficient endocytosis of Delta via the pushing force of WASp-Arp2/3 [
88], is consistent with the “pulling force” model for Notch activation that exposes the buried cleavage site of the extracellular Notch to eventually produce the NICD [
64,
65,
89].
4.2. Endocytosis in the Ligand-Independent Model of Notch Activation
Notch also participates in a ligand-independent, noncanonical activation through the activity of the ring finger ubiquitin ligase protein Deltex (Dx), which transports the Notch receptor from the cell surface towards the late endosomes [
70,
71,
81,
82]. Mono-ubiquitination of NICD by Dx blocks transport to MVB/ILVs and stabilizes NICD on maturing endosomes (the limiting membrane of the late endosome), which leads to Notch activation. Dx can also form a protein complex with the β-Arrestin Kurtz and the NICD. This complex switches NICD from mono- to poly-ubiquitination and targets Notch for degradation [
70,
71,
81,
82,
90]. Dx-mediated Notch activation is counteracted by the function of another ubiquitin ligase Suppressor of deltex (Su(dx)), which binds and internalizes NICD in clathrin-independent endocytosis (CIE) and promotes Notch transfer to ILVs of MVBs, late endosomes and degradation. Thus, Su(dx) and Dx compete with each other in order to direct Notch through these alternative routes in a context-dependent way [
71,
82]. Mutations in components of the endosomal–lysosomal sorting machinery were shown capable of triggering non-canonical signaling [
8,
27,
53,
65,
90]. For example, Shrub, a core component of the ESCRT-III complex, is a key regulator of the Dx and Kurtz interaction that promotes NICD endosomal/lysosomal degradation and delivery to MVBs [
81]. Shrub antagonizes Dx, enhances Kurtz activity and promotes the polyubiquitinated state of the Notch. However, it is the Notch mono- vs. poly-ubiquitination state that will determine the activation vs. degradation of Notch.
Other interesting studies shed light on how adaptor, scaffold or polarity determinants mediate the crosstalk between trafficking and junctional complexes as signaling centers. One example is Polychaetoid (Pyd), a negative regulator of the Dx-mediated Notch activation in the
Drosophila ovary stem cell niche and S2 cells [
82]. Pyd is the single
Drosophila homologue of the scaffolding zonula occludens-1 (ZO-1), junctional proteins that localize at tight junctions (TJs) in mammals. Pyd can bind and reduce Dx-dependent Notch trafficking, and thereby attenuate Notch signaling. Another interesting mechanism linking Notch to adaptor junctional components comes from the role of Crumbs in limiting ligand-independent endocytosis of Notch activation. In
Drosophila developing wings, the apical polarity determinant Crumbs binds Notch directly and prevents its activation through the non-canonical Dx-pathway. Crumbs exerts its function by directly binding and regulating Notch localization. Yet, this function is independent of the role of Crumbs in the localization of apical components as in other tissues [
82]. Taken together, Notch regulation is a very good example of the plethora of interactions where endocytosis and polarity networks converge with cell architecture and cell interactions to regulate signaling reception and recycling of Notch in order to fine-trim physiological signaling levels and cell fate decisions.