Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Medicine, Research & Experimental
Chimeric antigen receptor (CAR) T cells are artificially generated transgenic cells that express a hybrid in silico designed de novo dimeric immune receptor. The basic architecture of CAR receptors is an extracellular antigen recognition domain, a spacer domain, a transmembrane domain, and an intracellular signaling domain [35]. Each domain of a CAR receptor has been intensively studied and variations have been designed and established successfully.
- chimeric antigen receptor
1. Introduction
CAR T cell therapy has revolutionized immunotherapy in the last decade with the successful establishment of chimeric antigen receptor (CAR)-expressing cellular therapies as an alternative treatment in relapsed and refractory (r/r) homogeneously CD19-positive leukemias and lymphomas [1,2,3]. There are fundamental reasons why CAR T cell therapy has been approved by the Food and Drug administration (FDA) in the USA and the European Medicines Agency (EMA) for pediatric and young adult patients, as well as adult patients whose clinical data usually pave the way for translation of novel therapies into the clinic for children. Commonly, novel therapies are developed for the larger adult patient cohort, and then adapted for pediatric use, due to regulatory and commercial reasons [4,5]. Both strategic and biological factors have supported the development of CAR T cell therapy in children. The higher clinical relevance of CD19-positive malignancies in children compared to adults is one of the pivotal factors. B-cell acute lymphoblastic leukemia (B-ALL) is the most common pediatric malignancy, with a prevalence of up to 25% of cancers in all childhood cancers [6]. In contrast, the prevalence of all cancers in adults is below 0.5%, and B-cell non-Hodgkin’s lymphoma (NHL) represents approximately 3.6% of adult cancers [7,8]. Despite the unprecedented success story of ALL treatment in childhood, with 5 year overall survival rates exceeding 90% in contemporary treatment optimization studies [9], prognosis for r/r patients and patients with high-risk predispositions is still dismal [10]. Therefore, there is an urgent need for improved and more specific therapies in r/r ALL to reduce the adverse event profile and prolong survival. Furthermore, the susceptibility of B-ALL to CAR T cell therapy is significantly higher [2] than that of chronic lymphoblastic leukemia (CLL) [11] and a broad variety of B-lineage-derived lymphomas [12].
In general, pediatric ALL is an unmatched success story in cancer treatment, with high overall survival (OS) rates throughout the Western world, drastically increasing from no chance of survival in the 1950s, ~10% OS in the 1960s, ~40% OS in the 1970s, ~65% in the 1980s, to survival rates above 90% today [9]. The main reason for the excellent survival rates is the sophisticated chemotherapy protocols that have been initiated and optimized over the last seven decades [13]. Moreover, major advances have been achieved with the development and improvement of allogeneic hematopoietic stem cell transplantation (allo-HSCT) [14] and immunotherapy with the bispecific T cell engager therapy (BiTE) blinatumomab (CD3XCD19) [15,16], which is currently trialed in patients with precursor B-ALL as an alternative to conventional intensive and toxic chemotherapies, and in patients who are at high risk of relapse post chemotherapy in the clinical trial AIEOP-BFM ALL 2017 (NCT03643276).
CD19-CAR T cell therapy has been a medical breakthrough in the treatment of pediatric ALL, demonstrated by its outstanding clinical success, which exceeds previous therapies including allo-HSCT and blinatumomab treatment in r/r patients considered to be incurable with a shortened life expectancy [2,17]. CD19-targeted CAR-expressing T cells (CD19-CAR-T) were able to cure pediatric patients with a single-agent infusion trialed as the last resort after blinatumomab therapy [2]. Subsequent exploration of CD19-CAR-T cell treatment also demonstrated success in r/r ALL patients post allo-HSCT after infusion of true-allogeneic CD19-CAR T cells (donor-derived) [18] and pseudo-allogeneic (posttransplant recipient-derived) CD19-CAR T cells [19]. In the landmark clinical trials NCT01626495 and NCT01029366, autologous CD19-CAR-T treatment resulted in a high response rate (90% complete remission induction) and a 50% long-term event-free survival, despite recruitment of a limited number (N = 25) of patients [2]. These unprecedented clinical data in CAR T cell trials have led to the FDA approval of the first CD19-CAR-T cell therapy in children and young adults with B-ALL in 2017.
3. Molecular Architecture of CAR Receptors
CAR T cells are artificially generated transgenic cells that express a hybrid in silico designed de novo dimeric immune receptor. The basic architecture of CAR receptors is an extracellular antigen recognition domain, a spacer domain, a transmembrane domain, and an intracellular signaling domain [35]. Each domain of a CAR receptor has been intensively studied and variations have been designed and established successfully. It is noteworthy that critical steps in the development of CAR receptors were necessary to make CAR T cells potent therapeutics being capable of curing patients [36,37].
The main function and idea of CAR receptors are obviously to enable immune effector cells such as T cells and NK cells to be specifically redirected to cancer cells overexpressing the target antigen in a major histocompatibility complex (MHC)-independent manner [38,39]. scFv-based CAR receptors may also be constructed to target peptides presented by the MHC, for instance HLA-A2/NY-ESO-1 [40]. In Figure 1, the CAR architecture is illustrated and indicates established domain-variations.
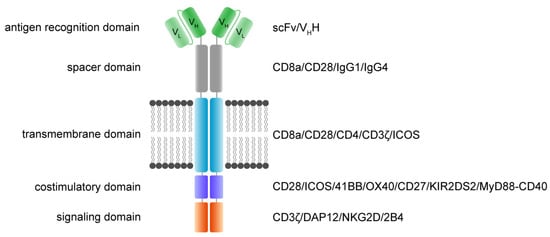
Figure 1. Functional modules of CAR receptors.
A schematic illustration of a second-generation CAR receptor. CAR receptors are comprised of several modules indicated in different colors—the antigen recognition domain, which usually consists of an antibody-derived scFv or VHH, the spacer domain of variable length, configuration, and flexibility, connecting the antigen recognition domain to the transmembrane domain. The transmembrane domain robustly anchors the CAR in the phospholipid bilayer cell membrane and is linked to the intracellular parts of the artificial immune receptor. Thus, another important role of the transmembrane domain is to facilitate the mechanic signal transduction into the cell. The intracellular costimulatory domains and signaling domain transform the activation signal via a signaling cascade into the cell to activate downstream signaling that results in various effector functions such as cytolysis, cytokine secretion and proliferation. scFv: single-chain variable fragment; VHH: heavy chain variable fragment of a single-domain antibody; VL: variable fragment of the light chain; VH: variable fragment of the heavy chain.
A CAR is a modular structure typically consisting of an extracellular antigen-binding domain linked by a spacer region to a transmembrane domain, attached to one or more intracellular activation domains. In general, every subunit of a CAR can significantly change the properties and function of the CAR receptor. CAR design has evolved over the last three decades, with the goal to improve CAR T cell efficacy, persistence, and safety.
The extracellular recognition domain in most CAR receptors is derived from the variable segments of the antibody light and heavy chains. They are constructed in line with peptide linkers [35,38] to assemble in a single-chain variable fragment (scFv) format. In general, scFvs are less stable in their configuration compared to the Fab region of antibodies [41]. Most antibodies in the past were generated by immunization of mice [42]. Today, fully human antibodies can be generated [43]. Single-domain VH binders (sdFv) based on human libraries or camelid binders or alternative formats can also be used as recognition domains [44]. The advantage of camelid sdFv is the reduced genetic load (half the size), reduced immunogenicity and the reduced tendency for aggregation while retaining the same specificity and affinity [45]. For hidden epitopes, the sdFv may be advantageous for the initial interaction of the targeted epitope compared to scFv based targeting due to less steric hinderance, higher solubility and the stability. Further, ligand-based CAR recognition domains have been introduced to target BCMA via trimeric APRIL [46], and the small chlorotoxin, a naturally derived 36-amino-acid-long peptide found in the venom of the death stalker scorpion leiurus quinquestriatus, which selectively binds to primary brain cancers is used for the treatment of glioblastoma (GBM) [47]. The basic requirement of recognition domains is the specific and rapid binding to the targeted antigen with the recognition domain to facilitate the CAR engagement.
The structural domains including the spacer (also called hinge) and transmembrane domains stabilize the receptor and allow the functional presentation of the recognition domain. They shape the extracellular configuration of the receptor and connect the extracellular domains to the intracellular modules of the receptor to facilitate an efficient mechanistic signal transduction to the intracellular signaling domains. Various protein subunits derived from CD8a, CD28, and IgG hinge regions also in combination with IgG CH2 and CH3 domains and others have been utilized as spacer domains, which have shown distinct properties. The most frequently used transmembrane domains are derived from the CD8a and CD28 [48].
The intracellular signaling domains usually contain one or more costimulatory domains and a signaling domain. Costimulatory domains are mainly derived from two families, namely the immunoglobulin superfamily, which is represented by CD28 and ICOS, and the tumor necrosis factor receptor superfamily (TNFR) represented by 4-1BB, OX40 and CD27. Signaling domains are mainly derived from the CD3ζ chain, while alternative signaling domains such as DAP12 have been used [49,50,51].
This entry is adapted from the peer-reviewed paper 10.3390/jcm11082158
This entry is offline, you can click here to edit this entry!
