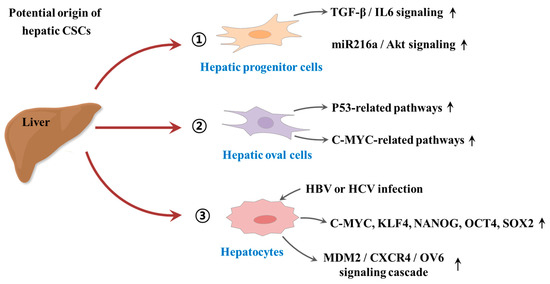
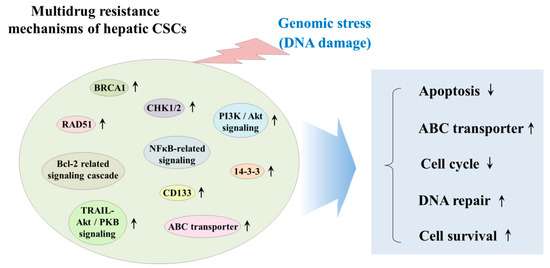
CSCs have been known to exhibit various genetic and/or epigenetic alternations that are associated with the resistance to classical therapeutic strategies, such as chemotherapy and radiotherapy [
71]. These various alternations include dysregulation of ATP-binding cassette (ABC) membrane transporters, cell cycle arrest (quiescent state), enhanced DNA repair efficiency, and high resistance to anticancer drug-induced apoptosis [
72]. Radiation and many types of chemotherapeutic agents exert their anticancer effects by inducing DNA damage to cancer cells; thus, it seems reasonable to hypothesize that the resistance of CSCs to classical therapeutic approaches may be due to the increased expression of DNA repair-related genes, such as BRCA1 and RAD51 [
73]. One of the most potent regulators of CSC resistance to DNA damaging chemotherapeutic drugs is DNA damage checkpoint protein kinases (CHKs), which are activated by genotoxic stress and delay the cell cycle progression to facilitate DNA repair [
74]. Lee et al. found that depletion of 14-3-3ζ, which regulates cell cycle, differentiation, and apoptosis, increases the sensitivity to radiation therapy in CD133
+ Huh7 liver cancer stem cells [
75]. Ma et al. reported that CD133
+ hepatic CSCs exhibit greater chemoresistance than CD133
− subpopulation by activating well-known pro-survival Akt/PKB and anti-apoptotic Bcl-2 signaling pathways [
76]. Another important regulator of the DNA repair systems against both endogenous and exogenous sources of DNA damage in stem cells is ATP-binding cassette transporters (ABC transporters), which can selectively extrude various toxic substrates, leading to multidrug resistance (MDR) [
77]. Indeed, Fung et al. found that enhanced expression levels of ABC transporters significantly promote chemoresistance, epithelial–mesenchymal transition (EMT) and cancer stemness in HCC model [
78]. PI3K/Akt, which is one of the most potent prosurvival signaling pathways, contributes to the maintenance and survival and also triggers endogenous drug resistance in CSCs [
79]. Indeed, Kahraman et al. showed PI3K/Akt/mTOR pathway-mediated resistance to Rapamycin to Sorafenib cotreatment in CD133
+/EpCAM
+ hepetic CSCs [
80]. Tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) plays an important role in cancer therapy by inducing selective apoptosis of cancer cells while having little effect on the normal cells [
81]. Zhu et al. reveal that TRAIL mediates drug resistance in various hepatic CSC models (PLC, HepG2 and Huh7 LC cells) through PI3K/Akt/Bad signaling cascades [
82]. Another promising target molecular to induce apoptosis in CSCs is nuclear factor kappa B (NFκB), which is known as an antiapoptotic signal transcription factor, can be activated by various chemodrugs including sorafenib [
83]. Zou et al. showed that sorafenib-induced NF-κB activation contributes to the enhanced resistance to sorafenib in CD133-positive sphere-forming hepatic CSCs [
84]. The multidrug resistance mechanisms of hepatic CSCs are summarized in .