Dipeptidyl peptidase IV (DPP-IV, CD26) is frequently dysregulated in cancer and plays an important role in regulating multiple bioactive peptides with the potential to influence cancer progression and the recruitment of immune cells. Therefore, it represents a potential contributing factor to cancer pathogenesis and an attractive therapeutic target. Specific DPP-IV inhibitors (gliptins) are currently used in patients with type 2 diabetes mellitus to promote insulin secretion by prolonging the activity of the incretins glucagon-like peptide 1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP). Nevertheless, the modulation of the bioavailability and function of other DPP-IV substrates, including chemokines, raises the possibility that the use of these orally administered drugs with favorable side-effect profiles might be extended beyond the treatment of hyperglycemia.
- gliptin
- cancer
- tumor microenvironment
- immune response
- chemokine
- stromal cell-derived factor
- drug repurposing
- stem cells
1. Introduction
2. DPP-IV Inhibition and Cancer Initiation and Progression
3. DPP-IV Inhibition in Anticancer Treatment
3.1. Direct Cytotoxic Effects of DPP-IV Inhibition on Cancer Cells
3.2. Effect of DPP-IV Inhibition on Anti-Tumor Immune Response
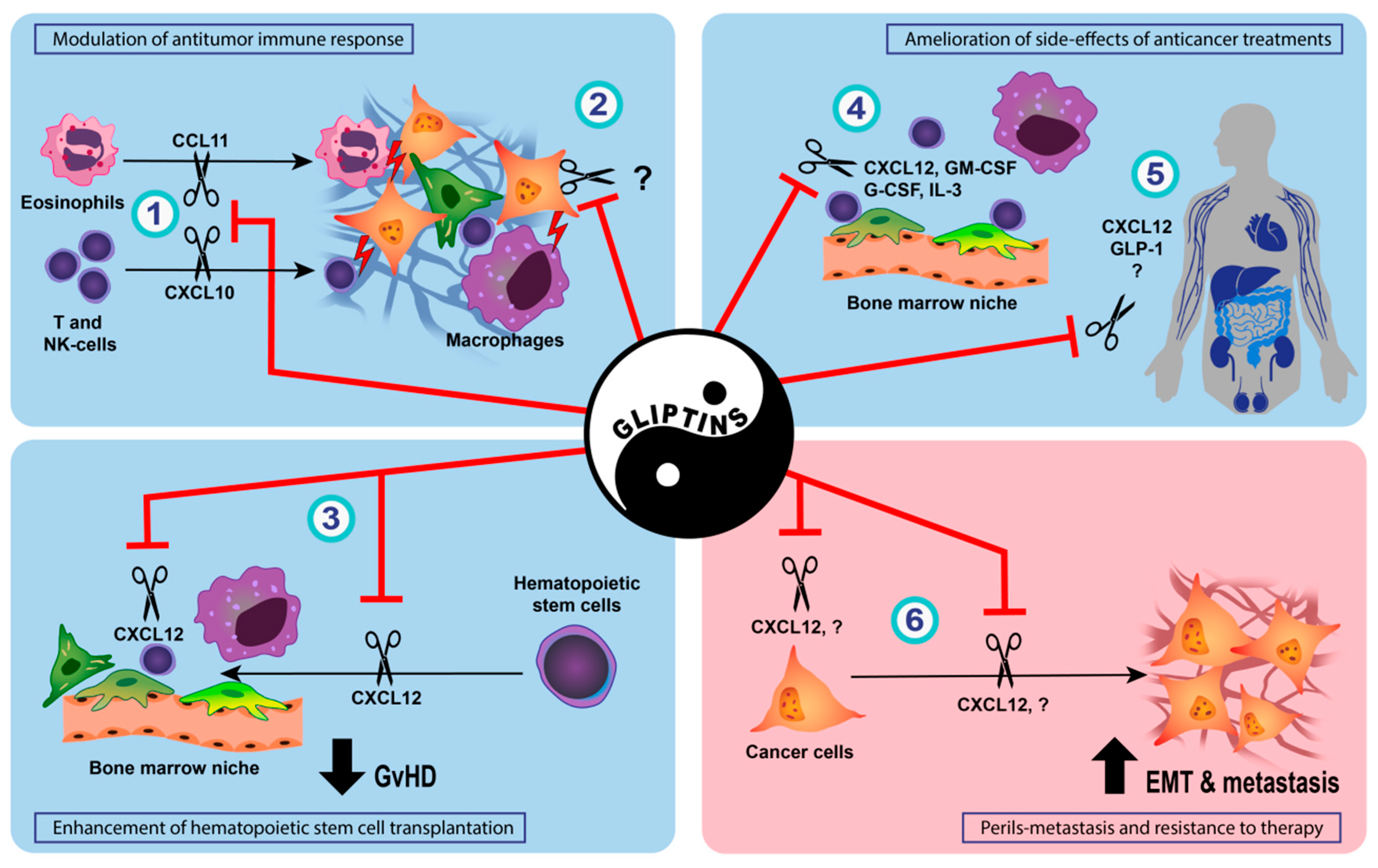
3.3. Enhancement of Hematopoietic Stem Cell Transplantation and Prophylaxis of Acute Graft-Versus-Host Disease by DPP-IV Inhibition
3.4. Amelioration of Side-Effects of Conventional Anticancer Treatments by DPP-IV Inhibition
This entry is adapted from the peer-reviewed paper 10.3390/cancers14092072
References
- Murgai, M.; Giles, A.; Kaplan, R. Physiological, Tumor, and Metastatic Niches: Opportunities and Challenges for Targeting the Tumor Microenvironment. Crit. Rev. Oncog. 2015, 20, 301–314.
- Busek, P.; Vanickova, Z.; Hrabal, P.; Brabec, M.; Fric, P.; Zavoral, M.; Skrha, J.; Kmochova, K.; Laclav, M.; Bunganic, B.; et al. Increased tissue and circulating levels of dipeptidyl peptidase-IV enzymatic activity in patients with pancreatic ductal adenocarcinoma. Pancreatology 2016, 16, 829–838.
- Matveyenko, A.V.; Dry, S.; Cox, H.I.; Moshtaghian, A.; Gurlo, T.; Galasso, R.; Butler, A.E.; Butler, P.C. Beneficial endocrine but adverse exocrine effects of sitagliptin in the human islet amyloid polypeptide transgenic rat model of type 2 diabetes: Interactions with metformin. Diabetes 2009, 58, 1604–1615.
- Butler, A.E.; Campbell-Thompson, M.; Gurlo, T.; Dawson, D.W.; Atkinson, M.; Butler, P.C. Marked expansion of exocrine and endocrine pancreas with incretin therapy in humans with increased exocrine pancreas dysplasia and the potential for glucagon-producing neuroendocrine tumors. Diabetes 2013, 62, 2595–2604.
- Ueberberg, S.; Jutte, H.; Uhl, W.; Schmidt, W.; Nauck, M.; Montanya, E.; Tannapfel, A.; Meier, J. Histological changes in endocrine and exocrine pancreatic tissue from patients exposed to incretin-based therapies. Diabetes Obes. Metab. 2016, 18, 1253–1262.
- Aston-Mourney, K.; Subramanian, S.L.; Zraika, S.; Samarasekera, T.; Meier, D.T.; Goldstein, L.C.; Hull, R.L. One year of sitagliptin treatment protects against islet amyloid-associated beta-cell loss and does not induce pancreatitis or pancreatic neoplasia in mice. Am. J. Physiol. Endocrinol. Metab. 2013, 305, E475–E484.
- Cox, A.R.; Lam, C.J.; Rankin, M.M.; Rios, J.S.; Chavez, J.; Bonnyman, C.W.; King, K.B.; Wells, R.A.; Anthony, D.; Tu, J.X.; et al. Incretin Therapies Do Not Expand beta-Cell Mass or Alter Pancreatic Histology in Young Male Mice. Endocrinology 2017, 158, 1701–1714.
- Busch, S.J.; Hoffmann, P.; Sahota, P.; Johnson, R.; Kothny, W.; Meyer, F.; Foley, J.E. Studies in rodents with the dipeptidyl peptidase-4 inhibitor vildagliptin to evaluate possible drug-induced pancreatic histological changes that are predictive of pancreatitis and cancer development in man. Diabetes Obes. Metab. 2013, 15, 72–76.
- Pinto, L.C.; Rados, D.V.; Barkan, S.S.; Leitao, C.B.; Gross, J.L. Dipeptidyl peptidase-4 inhibitors, pancreatic cancer and acute pancreatitis: A meta-analysis with trial sequential analysis. Sci. Rep. 2018, 8, 782.
- Abd El Aziz, M.; Cahyadi, O.; Meier, J.J.; Schmidt, W.E.; Nauck, M.A. Incretin-based glucose-lowering medications and the risk of acute pancreatitis and malignancies: A meta-analysis based on cardiovascular outcomes trials. Diabetes Obes. Metab. 2020, 22, 699–704.
- Dicembrini, I.; Montereggi, C.; Nreu, B.; Mannucci, E.; Monami, M. Pancreatitis and pancreatic cancer in patientes treated with Dipeptidyl Peptidase-4 inhibitors: An extensive and updated meta-analysis of randomized controlled trials. Diabetes Res. Clin. Pract. 2020, 159, 107981.
- Dicembrini, I.; Nreu, B.; Montereggi, C.; Mannucci, E.; Monami, M. Risk of cancer in patients treated with dipeptidyl peptidase-4 inhibitors: An extensive meta-analysis of randomized controlled trials. Acta Diabetol. 2020, 57, 689–696.
- Lee, D.Y.; Yu, J.H.; Park, S.; Han, K.; Kim, N.H.; Yoo, H.J.; Choi, K.M.; Baik, S.H.; Kim, N.H.; Seo, J.A. The influence of diabetes and antidiabetic medications on the risk of pancreatic cancer: A nationwide population-based study in Korea. Sci. Rep. 2018, 8, 9719.
- Boniol, M.; Franchi, M.; Bota, M.; Leclercq, A.; Guillaume, J.; van Damme, N.; Corrao, G.; Autier, P.; Boyle, P. Incretin-Based Therapies and the Short-term Risk of Pancreatic Cancer: Results From Two Retrospective Cohort Studies. Diabetes Care 2018, 41, 286–292.
- Lee, M.; Sun, J.; Han, M.; Cho, Y.; Lee, J.Y.; Nam, C.M.; Kang, E.S. Nationwide Trends in Pancreatitis and Pancreatic Cancer Risk Among Patients With Newly Diagnosed Type 2 Diabetes Receiving Dipeptidyl Peptidase 4 Inhibitors. Diabetes Care 2019, 42, 2057–2064.
- Abrahami, D.; Douros, A.; Yin, H.; Yu, O.H.; Faillie, J.L.; Montastruc, F.; Platt, R.W.; Bouganim, N.; Azoulay, L. Incretin based drugs and risk of cholangiocarcinoma among patients with type 2 diabetes: Population based cohort study. BMJ 2018, 363, k4880.
- Pech, V.; Abusaada, K.; Alemany, C. Dipeptidyl Peptidase-4 Inhibition May Stimulate Progression of Carcinoid Tumor. Case Rep. Endocrinol. 2015, 2015, 952019.
- Yang, F.; Takagaki, Y.; Yoshitomi, Y.; Ikeda, T.; Li, J.; Kitada, M.; Kumagai, A.; Kawakita, E.; Shi, S.; Kanasaki, K.; et al. Inhibition of Dipeptidyl Peptidase-4 Accelerates Epithelial-Mesenchymal Transition and Breast Cancer Metastasis via the CXCL12/CXCR4/mTOR Axis. Cancer Res. 2019, 79, 735–746.
- Kim, K.R.; Rhee, S.D.; Kim, H.Y.; Jung, W.H.; Yang, S.D.; Kim, S.S.; Ahn, J.H.; Cheon, H.G. KR-62436, 6-nicotinonitr ile, is a novel dipeptidyl peptidase-IV (DPP-IV) inhibitor with anti-hyperglycemic activity. Eur. J. Pharmacol. 2005, 518, 63–70.
- Russo, J.W.; Gao, C.; Bhasin, S.S.; Voznesensky, O.S.; Calagua, C.; Arai, S.; Nelson, P.S.; Montgomery, B.; Mostaghel, E.A.; Corey, E.; et al. Downregulation of Dipeptidyl Peptidase 4 Accelerates Progression to Castration-Resistant Prostate Cancer. Cancer Res. 2018, 78, 6354–6362.
- Wang, H.; Liu, X.; Long, M.; Huang, Y.; Zhang, L.; Zhang, R.; Zheng, Y.; Liao, X.; Wang, Y.; Liao, Q.; et al. NRF2 activation by antioxidant antidiabetic agents accelerates tumor metastasis. Sci. Transl. Med. 2016, 8, 334–351.
- He, L.; Zhang, T.; Sun, W.; Qin, Y.; Wang, Z.; Dong, W.; Zhang, H. The DPP-IV inhibitor saxagliptin promotes the migration and invasion of papillary thyroid carcinoma cells via the NRF2/HO1 pathway. Med. Oncol. 2020, 37, 97.
- Rathmann, W.; Kostev, K. Association of dipeptidyl peptidase 4 inhibitors with risk of metastases in patients with type 2 diabetes and breast, prostate or digestive system cancer. J. Diabetes Complicat. 2017, 31, 687–692.
- Noh, Y.; Jeon, S.M.; Shin, S. Association between glucose-lowering treatment and cancer metastasis among patients with preexisting type 2 diabetes and incident malignancy. Int. J. Cancer 2019, 144, 1530–1539.
- Tseng, C.H. Sitagliptin May Reduce Breast Cancer Risk in Women With Type 2 Diabetes. Clin. Breast Cancer 2017, 17, 211–218.
- Tseng, C.H. Sitagliptin may reduce prostate cancer risk in male patients with type 2 diabetes. Oncotarget 2017, 8, 19057–19064.
- Hsu, W.H.; Sue, S.P.; Liang, H.L.; Tseng, C.W.; Lin, H.C.; Wen, W.L.; Lee, M.Y. Dipeptidyl Peptidase 4 Inhibitors Decrease the Risk of Hepatocellular Carcinoma in Patients With Chronic Hepatitis C Infection and Type 2 Diabetes Mellitus: A Nationwide Study in Taiwan. Front. Public Health 2021, 9, 711723.
- Kamada, S.; Namekawa, T.; Ikeda, K.; Suzuki, T.; Kagawa, M.; Takeshita, H.; Yano, A.; Okamoto, K.; Ichikawa, T.; Horie-Inoue, K.; et al. Functional inhibition of cancer stemness-related protein DPP4 rescues tyrosine kinase inhibitor resistance in renal cell carcinoma. Oncogene 2021, 40, 3899–3913.
- Dietrich, P.; Wormser, L.; Fritz, V.; Seitz, T.; De Maria, M.; Schambony, A.; Kremer, A.E.; Gunther, C.; Itzel, T.; Thasler, W.E.; et al. Molecular crosstalk between Y5 receptor and neuropeptide Y drives liver cancer. J. Clin. Investig. 2020, 130, 2509–2526.
- Stecca, B.A.; Nardo, B.; Chieco, P.; Mazziotti, A.; Bolondi, L.; Cavallari, A. Aberrant dipeptidyl peptidase IV (DPP IV/CD26) expression in human hepatocellular carcinoma. J. Hepatol. 1997, 27, 337–345.
- Qin, C.J.; Zhao, L.H.; Zhou, X.; Zhang, H.L.; Wen, W.; Tang, L.; Zeng, M.; Wang, M.D.; Fu, G.B.; Huang, S.; et al. Inhibition of dipeptidyl peptidase IV prevents high fat diet-induced liver cancer angiogenesis by downregulating chemokine ligand 2. Cancer Lett. 2018, 420, 26–37.
- Kaji, K.; Yoshiji, H.; Ikenaka, Y.; Noguchi, R.; Aihara, Y.; Douhara, A.; Moriya, K.; Kawaratani, H.; Shirai, Y.; Yoshii, J.; et al. Dipeptidyl peptidase-4 inhibitor attenuates hepatic fibrosis via suppression of activated hepatic stellate cell in rats. J. Gastroenterol. 2014, 49, 481–491.
- Khalil, R.; Shata, A.; Abd El-Kader, E.M.; Sharaf, H.; Abdo, W.S.; Amin, N.A.; Saber, S. Vildagliptin, a DPP-4 inhibitor, attenuates carbon tetrachloride-induced liver fibrosis by targeting ERK1/2, p38alpha, and NF-kappaB signaling. Toxicol. Appl. Pharmacol. 2020, 407, 115246.
- Abd Elmaaboud, M.; Khattab, H.; Shalaby, S. Hepatoprotective effect of linagliptin against liver fibrosis induced by carbon tetrachloride in mice. Can. J. Physiol. Pharmacol. 2021, 99, 294–302.
- Sokar, S.S.; El-Sayad, M.E.; Ghoneim, M.E.; Shebl, A.M. Combination of Sitagliptin and Silymarin ameliorates liver fibrosis induced by carbon tetrachloride in rats. Biomed. Pharmacother. 2017, 89, 98–107.
- Wang, X.M.; Holz, L.E.; Chowdhury, S.; Cordoba, S.P.; Evans, K.A.; Gall, M.G.; de Ribeiro, A.J.V.; Zheng, Y.Z.; Levy, M.T.; Yu, D.M.; et al. The pro-fibrotic role of dipeptidyl peptidase 4 in carbon tetrachloride-induced experimental liver injury. Immunol. Cell Biol. 2017, 95, 443–453.
- Abd Elhameed, A.G.; Helal, M.G.; Said, E.; Salem, H.A. Saxagliptin defers thioacetamide-induced hepatocarcinogenesis in rats: A novel suppressive impact on Wnt/Hedgehog/Notch1 signaling. Environ. Toxicol. Pharmacol. 2021, 86, 103668.
- Nishina, S.; Yamauchi, A.; Kawaguchi, T.; Kaku, K.; Goto, M.; Sasaki, K.; Hara, Y.; Tomiyama, Y.; Kuribayashi, F.; Torimura, T.; et al. Dipeptidyl Peptidase 4 Inhibitors Reduce Hepatocellular Carcinoma by Activating Lymphocyte Chemotaxis in Mice. Cell. Mol. Gastroenterol. Hepatol. 2019, 7, 115–134.
- Baumeier, C.; Schluter, L.; Saussenthaler, S.; Laeger, T.; Rodiger, M.; Alaze, S.A.; Fritsche, L.; Haring, H.U.; Stefan, N.; Fritsche, A.; et al. Elevated hepatic DPP4 activity promotes insulin resistance and non-alcoholic fatty liver disease. Mol. Metab. 2017, 6, 1254–1263.
- Kawakubo, M.; Tanaka, M.; Ochi, K.; Watanabe, A.; Saka-Tanaka, M.; Kanamori, Y.; Yoshioka, N.; Yamashita, S.; Goto, M.; Itoh, M.; et al. Dipeptidyl peptidase-4 inhibition prevents nonalcoholic steatohepatitis-associated liver fibrosis and tumor development in mice independently of its anti-diabetic effects. Sci. Rep. 2020, 10, 983.
- Kawaguchi, T.; Nakano, D.; Koga, H.; Torimura, T. Effects of a DPP4 Inhibitor on Progression of NASH-related HCC and the p62/Keap1/Nrf2-Pentose Phosphate Pathway in a Mouse Model. Liver Cancer 2019, 8, 359–372.
- Ozutsumi, T.; Namisaki, T.; Shimozato, N.; Kaji, K.; Tsuji, Y.; Kaya, D.; Fujinaga, Y.; Furukawa, M.; Nakanishi, K.; Sato, S.; et al. Combined Treatment with Sodium-Glucose Cotransporter-2 Inhibitor (Canagliflozin) and Dipeptidyl Peptidase-4 Inhibitor (Teneligliptin) Alleviates NASH Progression in A Non-Diabetic Rat Model of Steatohepatitis. Int. J. Mol. Sci. 2020, 21, 2164.
- Okura, Y.; Namisaki, T.; Moriya, K.; Kitade, M.; Takeda, K.; Kaji, K.; Noguchi, R.; Nishimura, N.; Seki, K.; Kawaratani, H.; et al. Combined treatment with dipeptidyl peptidase-4 inhibitor (sitagliptin) and angiotensin-II type 1 receptor blocker (losartan) suppresses progression in a non-diabetic rat model of steatohepatitis. Hepatol. Res. Off. J. Jpn. Soc. Hepatol. 2017, 47, 1317–1328.
- Billeschou, A.; Hunt, J.E.; Ghimire, A.; Holst, J.J.; Kissow, H. Intestinal Adaptation upon Chemotherapy-Induced Intestinal Injury in Mice Depends on GLP-2 Receptor Activation. Biomedicines 2021, 9, 46.
- Masur, K.; Schwartz, F.; Entschladen, F.; Niggemann, B.; Zaenker, K.S. DPPIV inhibitors extend GLP-2 mediated tumour promoting effects on intestinal cancer cells. Regul. Pept. 2006, 137, 147–155.
- Abrahami, D.; Yin, H.; Yu, O.H.Y.; Pollak, M.N.; Azoulay, L. Incretin-based Drugs and the Incidence of Colorectal Cancer in Patients with Type 2 Diabetes. Epidemiology 2018, 29, 246–253.
- Ng, L.; Foo, D.C.; Wong, C.K.; Man, A.T.; Lo, O.S.; Law, W.L. Repurposing DPP-4 Inhibitors for Colorectal Cancer: A Retrospective and Single Center Study. Cancers 2021, 13, 3588.
- Ali, A.; Fuentes, A.; Skelton, W.I.; Wang, Y.; McGorray, S.; Shah, C.; Bishnoi, R.; Dang, L.H.; Dang, N.H. A multi-center retrospective analysis of the effect of DPP4 inhibitors on progression-free survival in advanced airway and colorectal cancers. Mol. Clin. Oncol. 2019, 10, 118–124.
- Bishnoi, R.; Hong, Y.R.; Shah, C.; Ali, A.; Skelton, W.P.t.; Huo, J.; Dang, N.H.; Dang, L.H. Dipeptidyl peptidase 4 inhibitors as novel agents in improving survival in diabetic patients with colorectal cancer and lung cancer: A Surveillance Epidemiology and Endpoint Research Medicare study. Cancer Med. 2019, 8, 3918–3927.
- Femia, A.P.; Raimondi, L.; Maglieri, G.; Lodovici, M.; Mannucci, E.; Caderni, G. Long-term treatment with Sitagliptin, a dipeptidyl peptidase-4 inhibitor, reduces colon carcinogenesis and reactive oxygen species in 1,2-dimethylhydrazine-induced rats. Int. J. Cancer 2013, 133, 2498–2503.
- Yorifuji, N.; Inoue, T.; Iguchi, M.; Fujiwara, K.; Kakimoto, K.; Nouda, S.; Okada, T.; Kawakami, K.; Abe, Y.; Takeuchi, T.; et al. The dipeptidyl peptidase-4 inhibitor sitagliptin suppresses mouse colon tumorigenesis in type 2 diabetic mice. Oncol. Rep. 2016, 35, 676–682.
- Fujiwara, K.; Inoue, T.; Henmi, Y.; Hirata, Y.; Naka, Y.; Hara, A.; Kakimoto, K.; Nouda, S.; Okada, T.; Kawakami, K.; et al. Sitagliptin, a dipeptidyl peptidase-4 inhibitor, suppresses CXCL5 and SDF-1 and does not accelerate intestinal neoplasia formation in Apc(Min/+) mice fed a high-fat diet. Oncol. Lett. 2017, 14, 4355–4360.
- Kabel, A.M.; Atef, A.; Estfanous, R.S. Ameliorative potential of sitagliptin and/or resveratrol on experimentally-induced clear cell renal cell carcinoma. Biomed. Pharmacother. 2018, 97, 667–674.
- Arwert, E.N.; Mentink, R.A.; Driskell, R.R.; Hoste, E.; Goldie, S.J.; Quist, S.; Watt, F.M. Upregulation of CD26 expression in epithelial cells and stromal cells during wound-induced skin tumour formation. Oncogene 2012, 31, 992–1000.
- Zhao, M.; Chen, J.; Yuan, Y.; Zou, Z.; Lai, X.; Rahmani, D.M.; Wang, F.; Xi, Y.; Huang, Q.; Bu, S. Dipeptidyl peptidase-4 inhibitors and cancer risk in patients with type 2 diabetes: A meta-analysis of randomized clinical trials. Sci. Rep. 2017, 7, 8273.
- Shirakawa, J.; Terauchi, Y. Potential linkage between dipeptidyl peptidase-4 inhibitor use and the risk of pancreatitis/pancreatic cancer. J. Diabetes Investig. 2020, 11, 789–791.
- Overbeek, J.A.; Bakker, M.; van der Heijden, A.; van Herk-Sukel, M.P.P.; Herings, R.M.C.; Nijpels, G. Risk of dipeptidyl peptidase-4 (DPP-4) inhibitors on site-specific cancer: A systematic review and meta-analysis. Diabetes Metab. Res. Rev. 2018, 34, e3004.
- Choi, Y.J.; Kim, D.J.; Shin, S. Incident cancer risk in dipeptidyl peptidase-4 inhibitor-treated patients with type 2 diabetes mellitus. Cancer Manag. Res. 2019, 11, 7427–7438.
- Jang, J.H.; Baerts, L.; Waumans, Y.; De Meester, I.; Yamada, Y.; Limani, P.; Gil-Bazo, I.; Weder, W.; Jungraithmayr, W. Suppression of lung metastases by the CD26/DPP4 inhibitor Vildagliptin in mice. Clin. Exp. Metastasis 2015, 32, 677–687.
- Kim, S.H.; Kang, J.G.; Kim, C.S.; Ihm, S.H.; Choi, M.G.; Yoo, H.J.; Lee, S.J. Synergistic cytotoxicity of the dipeptidyl peptidase-IV inhibitor gemigliptin with metformin in thyroid carcinoma cells. Endocrine 2018, 59, 383–394.
- Kim, S.H.; Kang, J.G.; Kim, C.S.; Ihm, S.H.; Choi, M.G.; Yoo, H.J.; Lee, S.J. Gemigliptin, a novel dipeptidyl peptidase-IV inhibitor, exerts a synergistic cytotoxicity with the histone deacetylase inhibitor PXD101 in thyroid carcinoma cells. J. Endocrinol. Investig. 2018, 41, 677–689.
- Kim, S.H.; Kang, J.G.; Kim, C.S.; Ihm, S.H.; Choi, M.G.; Yoo, H.J.; Lee, S.J. The dipeptidyl peptidase-IV inhibitor gemigliptin alone or in combination with NVP-AUY922 has a cytotoxic activity in thyroid carcinoma cells. Tumour Biol. 2017, 39, 1010428317722068.
- Lee, J.J.; Wang, T.Y.; Liu, C.L.; Chien, M.N.; Chen, M.J.; Hsu, Y.C.; Leung, C.H.; Cheng, S.P. Dipeptidyl Peptidase IV as a Prognostic Marker and Therapeutic Target in Papillary Thyroid Carcinoma. J. Clin. Endocrinol. Metab. 2017, 102, 2930–2940.
- Wang, Q.; Lu, P.; Wang, T.; Zheng, Q.; Li, Y.; Leng, S.X.; Meng, X.; Wang, B.; Xie, J.; Zhang, H. Sitagliptin affects gastric cancer cells proliferation by suppressing Melanoma-associated antigen-A3 expression through Yes-associated protein inactivation. Cancer Med. 2020, 9, 3816–3828.
- Choi, H.J.; Kim, J.Y.; Lim, S.C.; Kim, G.; Yun, H.J.; Choi, H.S. Dipeptidyl peptidase 4 promotes epithelial cell transformation and breast tumourigenesis via induction of PIN1 gene expression. Br. J. Pharmacol. 2015, 172, 5096–5109.
- Yang, X.; Zhang, X.; Wu, R.; Huang, Q.; Jiang, Y.; Qin, J.; Yao, F.; Jin, G.; Zhang, Y. DPPIV promotes endometrial carcinoma cell proliferation, invasion and tumorigenesis. Oncotarget 2017, 8, 8679–8692.
- Varela-Calvino, R.; Rodriguez-Quiroga, M.; Carvalho, P.D.; Martins, F.; Serra-Roma, A.; Vazquez-Iglesias, L.; de la Cadena, M.P.; Velho, S.; Cordero, O.J. The mechanism of sitagliptin inhibition of colorectal cancer cell lines’ metastatic functionalities. IUBMB Life 2021, 73, 761–773.
- Bergman, A.J.; Stevens, C.; Zhou, Y.; Yi, B.; Laethem, M.; De Smet, M.; Snyder, K.; Hilliard, D.; Tanaka, W.; Zeng, W.; et al. Pharmacokinetic and pharmacodynamic properties of multiple oral doses of sitagliptin, a dipeptidyl peptidase-IV inhibitor: A double-blind, randomized, placebo-controlled study in healthy male volunteers. Clin. Ther. 2006, 28, 55–72.
- Herrmann, H.; Sadovnik, I.; Cerny-Reiterer, S.; Rulicke, T.; Stefanzl, G.; Willmann, M.; Hoermann, G.; Bilban, M.; Blatt, K.; Herndlhofer, S.; et al. Dipeptidylpeptidase IV (CD26) defines leukemic stem cells (LSC) in chronic myeloid leukemia. Blood 2014, 123, 3951–3962.
- Willmann, M.; Sadovnik, I.; Eisenwort, G.; Entner, M.; Bernthaler, T.; Stefanzl, G.; Hadzijusufovic, E.; Berger, D.; Herrmann, H.; Hoermann, G.; et al. Evaluation of cooperative antileukemic effects of nilotinib and vildagliptin in Ph(+) chronic myeloid leukemia. Exp. Hematol. 2018, 57, 50–59.e56.
- Hollande, C.; Boussier, J.; Ziai, J.; Nozawa, T.; Bondet, V.; Phung, W.; Lu, B.; Duffy, D.; Paradis, V.; Mallet, V.; et al. Inhibition of the dipeptidyl peptidase DPP4 (CD26) reveals IL-33-dependent eosinophil-mediated control of tumor growth. Nat. Immunol. 2019, 20, 257–264.
- Sliwinska, A.; Rogalska, A.; Marczak, A.; Kasznicki, J.; Drzewoski, J. Metformin, but not sitagliptin, enhances WP 631-induced apoptotic HepG2 cell death. Toxicol. Vitr. 2015, 29, 1116–1123.
- Kawaguchi, T.; Kodama, T.; Hikita, H.; Makino, Y.; Saito, Y.; Tanaka, S.; Shimizu, S.; Sakamori, R.; Miyagi, T.; Wada, H.; et al. Synthetic lethal interaction of combined CD26 and Bcl-xL inhibition is a powerful anticancer therapy against hepatocellular carcinoma. Hepatol. Res. Off. J. Jpn. Soc. Hepatol. 2015, 45, 1023–1033.
- Spagnuolo, P.A.; Hurren, R.; Gronda, M.; MacLean, N.; Datti, A.; Basheer, A.; Lin, F.H.; Wang, X.; Wrana, J.; Schimmer, A.D. Inhibition of intracellular dipeptidyl peptidases 8 and 9 enhances parthenolide’s anti-leukemic activity. Leukemia 2013, 27, 1236–1244.
- Sato, T.; Tatekoshi, A.; Takada, K.; Iyama, S.; Kamihara, Y.; Jawaid, P.; Rehman, M.U.; Noguchi, K.; Kondo, T.; Kajikawa, S.; et al. DPP8 is a novel therapeutic target for multiple myeloma. Sci. Rep. 2019, 9, 18094.
- Beckenkamp, A.; Willig, J.B.; Santana, D.B.; Nascimento, J.; Paccez, J.D.; Zerbini, L.F.; Bruno, A.N.; Pilger, D.A.; Wink, M.R.; Buffon, A. Differential Expression and Enzymatic Activity of DPPIV/CD26 Affects Migration Ability of Cervical Carcinoma Cells. PLoS ONE 2015, 10, e0134305.
- Yamaguchi, T.; Watanabe, A.; Tanaka, M.; Shiota, M.; Osada-Oka, M.; Sano, S.; Yoshiyama, M.; Miura, K.; Kitajima, S.; Matsunaga, S.; et al. A dipeptidyl peptidase-4 (DPP-4) inhibitor, linagliptin, attenuates cardiac dysfunction after myocardial infarction independently of DPP-4. J. Pharmacol. Sci. 2019, 139, 112–119.
- Batchu, S.N.; Yerra, V.G.; Liu, Y.; Advani, S.L.; Klein, T.; Advani, A. The Dipeptidyl Peptidase-4 Inhibitor Linagliptin Directly Enhances the Contractile Recovery of Mouse Hearts at a Concentration Equivalent to that Achieved with Standard Dosing in Humans. Int. J. Mol. Sci. 2020, 21, 5756.
- Heo, R.; Seo, M.S.; An, J.R.; Kang, M.; Park, H.; Han, E.T.; Han, J.H.; Chun, W.; Park, W.S. The anti-diabetic drug trelagliptin induces vasodilation via activation of Kv channels and SERCA pumps. Life Sci. 2021, 283, 119868.
- Bailey, S.R.; Nelson, M.H.; Majchrzak, K.; Bowers, J.S.; Wyatt, M.M.; Smith, A.S.; Neal, L.R.; Shirai, K.; Carpenito, C.; June, C.H.; et al. Human CD26(high) T cells elicit tumor immunity against multiple malignancies via enhanced migration and persistence. Nat. Commun. 2017, 8, 1961.
- Nelson, M.H.; Knochelmann, H.M.; Bailey, S.R.; Huff, L.W.; Bowers, J.S.; Majchrzak-Kuligowska, K.; Wyatt, M.M.; Rubinstein, M.P.; Mehrotra, S.; Nishimura, M.I.; et al. Identification of human CD4(+) T cell populations with distinct antitumor activity. Sci. Adv. 2020, 6, eaba7443.
- Casrouge, A.; Decalf, J.; Ahloulay, M.; Lababidi, C.; Mansour, H.; Vallet-Pichard, A.; Mallet, V.; Mottez, E.; Mapes, J.; Fontanet, A.; et al. Evidence for an antagonist form of the chemokine CXCL10 in patients chronically infected with HCV. J. Clin. Investig. 2011, 121, 308–317.
- da Silva, R.B.; Laird, M.E.; Yatim, N.; Fiette, L.; Ingersoll, M.A.; Albert, M.L. Dipeptidylpeptidase 4 inhibition enhances lymphocyte trafficking, improving both naturally occurring tumor immunity and immunotherapy. Nat. Immunol. 2015, 16, 850–858.
- Cook, S.J.; Lee, Q.; Wong, A.C.; Spann, B.C.; Vincent, J.N.; Wong, J.J.; Schlitzer, A.; Gorrell, M.D.; Weninger, W.; Roediger, B. Differential chemokine receptor expression and usage by pre-cDC1 and pre-cDC2. Immunol. Cell Biol. 2018, 96, 1131–1139.
- Wilson, A.L.; Moffitt, L.R.; Wilson, K.L.; Bilandzic, M.; Wright, M.D.; Gorrell, M.D.; Oehler, M.K.; Plebanski, M.; Stephens, A.N. DPP4 Inhibitor Sitagliptin Enhances Lymphocyte Recruitment and Prolongs Survival in a Syngeneic Ovarian Cancer Mouse Model. Cancers 2021, 13, 487.
- Jang, J.H.; Janker, F.; De Meester, I.; Arni, S.; Borgeaud, N.; Yamada, Y.; Gil Bazo, I.; Weder, W.; Jungraithmayr, W. The CD26/DPP4-inhibitor vildagliptin suppresses lung cancer growth via macrophage-mediated NK cell activity. Carcinogenesis 2019, 40, 324–334.
- Christopherson, K.W., 2nd; Hangoc, G.; Mantel, C.R.; Broxmeyer, H.E. Modulation of hematopoietic stem cell homing and engraftment by CD26. Science 2004, 305, 1000–1003.
- Farag, S.S.; Srivastava, S.; Messina-Graham, S.; Schwartz, J.; Robertson, M.J.; Abonour, R.; Cornetta, K.; Wood, L.; Secrest, A.; Strother, R.M.; et al. In vivo DPP-4 inhibition to enhance engraftment of single-unit cord blood transplants in adults with hematological malignancies. Stem Cells Dev. 2013, 22, 1007–1015.
- Farag, S.S.; Nelson, R.; Cairo, M.S.; O’Leary, H.A.; Zhang, S.; Huntley, C.; Delgado, D.; Schwartz, J.; Zaid, M.A.; Abonour, R.; et al. High-dose sitagliptin for systemic inhibition of dipeptidylpeptidase-4 to enhance engraftment of single cord umbilical cord blood transplantation. Oncotarget 2017, 8, 110350–110357.
- Farag, S.S.; Abu Zaid, M.; Schwartz, J.E.; Thakrar, T.C.; Blakley, A.J.; Abonour, R.; Robertson, M.J.; Broxmeyer, H.E.; Zhang, S. Dipeptidyl Peptidase 4 Inhibition for Prophylaxis of Acute Graft-versus-Host Disease. N. Engl. J. Med. 2021, 384, 11–19.
- Broxmeyer, H.E.; Hoggatt, J.; O’Leary, H.A.; Mantel, C.; Chitteti, B.R.; Cooper, S.; Messina-Graham, S.; Hangoc, G.; Farag, S.; Rohrabaugh, S.L.; et al. Dipeptidylpeptidase 4 negatively regulates colony-stimulating factor activity and stress hematopoiesis. Nat. Med. 2012, 18, 1786–1796.
- Iwakura, T.; Zhao, Z.; Marschner, J.A.; Devarapu, S.K.; Yasuda, H.; Anders, H.J. Dipeptidyl peptidase-4 inhibitor teneligliptin accelerates recovery from cisplatin-induced acute kidney injury by attenuating inflammation and promoting tubular regeneration. Nephrol. Dial. Transplant. 2019, 34, 1669–1680.
- Salama, R.M.; Nasr, M.M.; Abdelhakeem, J.I.; Roshdy, O.K.; ElGamal, M.A. Alogliptin attenuates cyclophosphamide-induced nephrotoxicity: A novel therapeutic approach through modulating MAP3K/JNK/SMAD3 signaling cascade. Drug Chem. Toxicol. 2020, 45, 1254–1263.
- Jo, C.H.; Kim, S.; Park, J.S.; Kim, G.H. Anti-Inflammatory Action of Sitagliptin and Linagliptin in Doxorubicin Nephropathy. Kidney Blood Press. Res. 2018, 43, 987–999.
- Mostafa, R.E.; Morsi, A.H.; Asaad, G.F. Anti-inflammatory effects of saxagliptin and vildagliptin against doxorubicin-induced nephrotoxicity in rats: Attenuation of NLRP3 inflammasome up-regulation and tubulo-interstitial injury. Res. Pharm. Sci. 2021, 16, 547–558.
- Iwakura, T.; Fukasawa, H.; Kitamura, A.; Ishibuchi, K.; Yasuda, H.; Furuya, R. Effect of dipeptidyl peptidase-4 inhibitors on cisplatin-induced acute nephrotoxicity in cancer patients with diabetes mellitus: A retrospective study. PLoS ONE 2020, 15, e0229377.
- El-Agamy, D.S.; Abo-Haded, H.M.; Elkablawy, M.A. Cardioprotective effects of sitagliptin against doxorubicin-induced cardiotoxicity in rats. Exp. Biol. Med. 2016, 241, 1577–1587.
- Aykan, D.A.; Yaman, S.; Eser, N.; Ozcan Metin, T.; Seyithanoglu, M.; Aykan, A.C.; Kurt, A.H.; Ergun, Y. Bisoprolol and linagliptin ameliorated electrical and mechanical isometric myocardial contractions in doxorubicin-induced cardiomyopathy in rats. Pharmacol. Rep. 2020, 72, 867–876.
- Shigematsu, N.; Kawashiri, T.; Kobayashi, D.; Shimizu, S.; Mine, K.; Hiromoto, S.; Uchida, M.; Egashira, N.; Shimazoe, T. Neuroprotective effect of alogliptin on oxaliplatin-induced peripheral neuropathy in vivo and in vitro. Sci. Rep. 2020, 10, 6734.
- Abo-Haded, H.M.; Elkablawy, M.A.; Al-Johani, Z.; Al-Ahmadi, O.; El-Agamy, D.S. Hepatoprotective effect of sitagliptin against methotrexate induced liver toxicity. PLoS ONE 2017, 12, e0174295.
- Elrashidy, R.A.; Hasan, R.A. Stromal cell-derived factor-1alpha predominantly mediates the ameliorative effect of linagliptin against cisplatin-induced testicular injury in adult male rats. Cytokine 2020, 136, 155260.
- Lee, J.M.; Yoo, I.K.; Lee, J.M.; Kim, S.H.; Choi, H.S.; Kim, E.S.; Keum, B.; Seo, Y.S.; Jeen, Y.T.; Chun, H.J.; et al. Dipeptidyl-peptidase-4 (DPP-4) inhibitor ameliorates 5-flurouracil induced intestinal mucositis. BMC Cancer 2019, 19, 1016.
- Narducci, M.G.; Scala, E.; Bresin, A.; Caprini, E.; Picchio, M.C.; Remotti, D.; Ragone, G.; Nasorri, F.; Frontani, M.; Arcelli, D.; et al. Skin homing of Sezary cells involves SDF-1-CXCR4 signaling and down-regulation of CD26/dipeptidylpeptidase IV. Blood 2006, 107, 1108–1115.
- Sun, Y.X.; Pedersen, E.A.; Shiozawa, Y.; Havens, A.M.; Jung, Y.; Wang, J.; Pienta, K.J.; Taichman, R.S. CD26/dipeptidyl peptidase IV regulates prostate cancer metastasis by degrading SDF-1/CXCL12. Clin. Exp. Metastasis 2008, 25, 765–776.
- Proost, P.; Schutyser, E.; Menten, P.; Struyf, S.; Wuyts, A.; Opdenakker, G.; Detheux, M.; Parmentier, M.; Durinx, C.; Lambeir, A.M.; et al. Amino-terminal truncation of CXCR3 agonists impairs receptor signaling and lymphocyte chemotaxis, while preserving antiangiogenic properties. Blood 2001, 98, 3554–3561.
- Kuo, L.E.; Abe, K.; Zukowska, Z. Stress, NPY and vascular remodeling: Implications for stress-related diseases. Peptides 2007, 28, 435–440.
- Yamamoto, S.; Tokuhara, T.; Nishikawa, M.; Nishizawa, S.; Nishioka, T.; Nozawa, A.; Takahashi, A.; Watanabe, Y.; Wada, R.; Wakasa, K.; et al. Spontaneous regression of hepatocellular carcinoma after improving diabetes mellitus: Possibly responsible for immune system. Kanzo 2012, 53, 164–174.
- Duarte, R.F.; Labopin, M.; Bader, P.; Basak, G.W.; Bonini, C.; Chabannon, C.; Corbacioglu, S.; Dreger, P.; Dufour, C.; Gennery, A.R.; et al. Indications for haematopoietic stem cell transplantation for haematological diseases, solid tumours and immune disorders: Current practice in Europe, 2019. Bone Marrow Transplant. 2019, 54, 1525–1552.
- Sugiyama, T.; Kohara, H.; Noda, M.; Nagasawa, T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity 2006, 25, 977–988.
- Peled, A.; Petit, I.; Kollet, O.; Magid, M.; Ponomaryov, T.; Byk, T.; Nagler, A.; Ben-Hur, H.; Many, A.; Shultz, L.; et al. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science 1999, 283, 845–848.
- Proost, P.; Struyf, S.; Schols, D.; Durinx, C.; Wuyts, A.; Lenaerts, J.P.; De Clercq, E.; De Meester, I.; Van Damme, J. Processing by CD26/dipeptidyl-peptidase IV reduces the chemotactic and anti-HIV-1 activity of stromal-cell-derived factor-1alpha. FEBS Lett. 1998, 432, 73–76.
- Schwaiger, E.; Klaus, C.; Matheeussen, V.; Baranyi, U.; Pilat, N.; Ramsey, H.; Korom, S.; De Meester, I.; Wekerle, T. Dipeptidyl peptidase IV (DPPIV/CD26) inhibition does not improve engraftment of unfractionated syngeneic or allogeneic bone marrow after nonmyeloablative conditioning. Exp. Hematol. 2012, 40, 97–106.
- Kawai, T.; Choi, U.; Liu, P.C.; Whiting-Theobald, N.L.; Linton, G.F.; Malech, H.L. Diprotin A infusion into nonobese diabetic/severe combined immunodeficiency mice markedly enhances engraftment of human mobilized CD34+ peripheral blood cells. Stem Cells Dev. 2007, 16, 361–370.
- Mulvihill, E.E.; Drucker, D.J. Pharmacology, physiology, and mechanisms of action of dipeptidyl peptidase-4 inhibitors. Endocr. Rev. 2014, 35, 992–1019.
- Klemann, C.; Wagner, L.; Stephan, M.; von Horsten, S. Cut to the chase: A review of CD26/dipeptidyl peptidase-4’s (DPP4) entanglement in the immune system. Clin. Exp. Immunol. 2016, 185, 1–21.
- Busek, P.; Sedo, A. Dipeptidyl Peptidase-IV and Related Proteases in Brain Tumors. In Evolution of the Molecular Biology of Brain Tumors and the Therapeutic Implications; Lichtor, T., Ed.; InTech: London, UK, 2013.
- Lambeir, A.M.; Durinx, C.; Scharpe, S.; De Meester, I. Dipeptidyl-peptidase IV from bench to bedside: An update on structural properties, functions, and clinical aspects of the enzyme DPP IV. Crit. Rev. Clin. Lab. Sci. 2003, 40, 209–294.
- Mentlein, R. Dipeptidyl-peptidase IV (CD26)—Role in the inactivation of regulatory peptides. Regul. Pept. 1999, 85, 9–24.
- Kissow, H.; Hartmann, B.; Holst, J.J.; Poulsen, S.S. Glucagon-like peptide-1 as a treatment for chemotherapy-induced mucositis. Gut 2013, 62, 1724–1733.
- Muscogiuri, G.; DeFronzo, R.A.; Gastaldelli, A.; Holst, J.J. Glucagon-like Peptide-1 and the Central/Peripheral Nervous System: Crosstalk in Diabetes. Trends Endocrinol. Metab. 2017, 28, 88–103.
- Davidson, E.P.; Coppey, L.J.; Dake, B.; Yorek, M.A. Treatment of streptozotocin-induced diabetic rats with alogliptin: Effect on vascular and neural complications. Exp. Diabetes Res. 2011, 2011, 810469.
- De La Vega, M.R.; Chapman, E.; Zhang, D.D. NRF2 and the Hallmarks of Cancer. Cancer Cell 2018, 34, 21–43.
