Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Male breast cancer (MBC) is a rare disease. Genetic factors predispose to male breast cancer. Germline and/or genetic and/or epigenetic alterations at the somatic level identify a subset of male breast cancer that could differ from female breast cancer (FBC).
- male breast cancer
- BRCA1/2
1. Introduction
MBC is a rare disease [1]. Current evidence about treatment is derived from small single institutional experiences. The knowledge of genetic drivers of MBC could drive prospective clinical trials of more specific and targeted therapies.
2. Management
2.1. Imaging
MBC patients usually presents with signs of locally advanced tumor (nipple and/or skin involvement); this is due to the smaller breast size in men, and probably the later manifestation of breast symptoms. MBC is commonly located in the subareolar region while in FBC, it is commonly in the upper-outer quadrant. Malignant calcifications are less frequent in men than in women, and their mammographic features (i.e., scattered and punctuate) would be considered benign in women. Cystic lesions are rare, which is different from what occurs in women, and they should be considered as suspicious of malignancy. Lobular tissue is not commonly present in male breasts and for this reason cysts are uncommon. For instance, in men papillary carcinomas are generally detected as cysts with a complex pattern at US-imaging. MBC can be erroneously diagnosed for asymmetric/unilateral gynecomastia; however, the first is often eccentric to the nipple while the latter is central and concentric to the nipple [2].
2.2. Histopathology
The most frequent histology of MBCs is invasive ductal carcinoma, which occurs in 85–95% of patients. Ductal carcinoma in situ is diagnosed in 5–10% of male with BC [1].
Regarding immunohistochemistry phenotypes, MBC seems to be more likely to express hormone receptors than FBC. In small retrospective studies, 82% of the included MBCs were positive for estrogen receptors (ER)s and 75% showed positivity for progestin receptors (PRs) [1]. A recent large retrospective analysis of 489 male patients confirmed these findings [3].
ERs were evaluable in 419 tumors, with 92% of tumors being positive; PR status was assessable in 399 tumors and was positive in 89.2% of cases. The concurrent evaluation of both hormone receptors shows ER+/PR+ = 86%; ER+/PR− = 6%; ER−/PR+ = 3.3%; and ER−/PR− = 4.8%. More than 95% of the tumors showed positivity in at least one hormone receptor. However, tumor subtypes seem to be distributed differently from that seen for women and varies according to race/ethnicity. In fact, as reported by Mc Gregor and colleagues, among 606 patients with different ethnic origins triple-negative or ER-positive/PR-negative tumors are more frequent in non-Hispanic black men for which is also reported a poorer outcome [4].
In the EORTC International Male Breast Cancer Program, 1483 MBCs were retrospectively analyzed over 20 years. For tumors without metastases, ductal invasive carcinomas were reported in 84.8% of cases, with grade 2 in 51.5%; ER-positivity in 99.3%; PR positivity in 81.9%; AR positivity in 96.9% and low expression of Ki67 in 61.1%. The most common subtypes in MBC were the luminal A-like (41.9%) and luminal B-like/HER-2-negative (48.6%), whereas less frequent were the HER-2-positive (8.7%) and triple-negative (0.3%) subtypes [5]. Men aged less than 50 years old had poorer outcomes. In tumors with high ER positivity, with high PR positivity, and high AR positivity, significantly longer overall survival (OS) and recurrent-free survival (RFS) was reported. While HER2 expression, Ki67, IHC subtypes or grade were no associated to OS/RFS [6].
Gargiulo et al. also reported a high prevalence of positive hormonal receptor status (88.4% ER+; 81.4% PR+) in MBC, with HER2-positive and triple-negative tumor prevalence being 26.8% and 7.0%, respectively [7].
HER2 expression has been reported to range between 2% and 27% in MBC. Humphries et al. showed a very low expression of HER2 in MBC, accounting for <10%. In the series by Gargiulo et al., a higher expression was reported, probably attributable to the non-systematic evaluation [1][7][8].
Other biomarkers were recently evaluated in 134 MBCs. Cyclin D1 and bcl-2 are frequently expressed (75% and 77%, respectively) in MBCs. BRST2 and p21 are expressed in 56% and 48% of MBCs, respectively; p53 is expressed only in 15% of these tumors and basal phenotype is uncommon [6]. Furthermore, it has been demonstrated that there is an association between high grade, high mitotic count and HER2 amplification and/or overexpression, high Ki67, p53 accumulation, high p21 expression, low PR expression, and low bcl-2 expression. A decreased 5-years survival were statistically associated to PR negativity and p53 accumulation; they were also independent markers of patient prognosis [9].
Histopathological features seem to be related to the tumor mutational status. An Italian multicenter study evaluating 382 male patients with BCs, including 50 BRCA carriers, reported that MBCs in BRCA2 carriers showed a statistically higher tumor grade, PR negativity, and HER2 positivity. Carriers of BRCA2 pathogenetic variants develop more frequently secondary primary tumors (OR = 11.42, 95% CI 1.79–73.08). BRCA2-related MBCs have a specific phenotype characterized by high-grade tumors, PR-negative status and consequently a more aggressive behavior [10].
MBC is often diagnosed at an advanced stage. T4 disease represented 20–25% of cancers [11]. The pT3–T4 stage significantly increases according to age, with the highest percentage (42%) in men over 70 years old [11]. Axillary nodal involvement is present in approximately 50% of MBC [3][11][12] and significantly correlates with the pathological tumor size [3].
2.3. Treatments
Treatment options, schedules, and duration of therapies in MBC for both localized and metastatic disease are usually derived from recommendations and guidelines for FBC. Treatment includes the integration of surgery, radiotherapy, and systemic therapies.
Modified radical mastectomy is the preferred surgical approach for MBCs, used in approximately 70% of patients. Less favored approaches are represented by radical mastectomy, especially in older patients, total mastectomy, and lumpectomy with or without radiation [11]. Sarmiento et al. reported, in a large population-based cohort, an evaluation on 16,498 MBCs from the National Cancer Database and showed an improved survival related to treatments, particularly surgery. Increasing age, black race, government insurance, more comorbidities, and higher tumor stages were associated with decreased survival [12]. More recently, Yadav et al. confirmed the association between a worse prognosis and advanced age, black ethnicity, comorbidities, high grade and stages, and poor access to health care [13]. They reported a negative association with mastectomy, differently from the study of Sarmiento. Yadav et al. found that more male patients underwent total mastectomy compared to breast-conserving treatment, which is the preferred option for surgical treatment in females. They showed an association between radical mastectomy and poor clinical outcomes, possibly due to a selection bias because of higher stage associated to larger tumors size and/or node involvement in this cohort. Further evaluation is needed [14].
Moreover, in MBC, like in FBC, sentinel lymph node (SLN) biopsy is a reliable tool for the identification of nodal metastases, as shown by two different experiences in a European and USA Center [15][16].
No strong evidence exists on the use of radiation after mastectomy. Current recommendations suggest adhering to the guidelines for FBC, as shown by a recent single institutional experience [1][17].
Hormonal therapy (HT) represents the gold standard treatment for hormone receptor-positive MBC. Adjuvant HT is represented by tamoxifen for 5 years. Tamoxifen is associated to a decreased risk of recurrence, accounting by 51%, comparable with FBC treatment [18]. In male, about 80% of the circulating estrogen is produced by the aromatase pathway and 20% by the testes [19]. However, the role of adjuvant aromatase inhibitors (AIs) has been little studied in male patients [20]. Eggermann et al., compared adjuvant tamoxifen vs AIs [21].They reported a statistically significant worse outcome in AI cohort for both mortality risk and overall survival [21].
In high-risk patients because of young age, high tumor grade and/or axillary nodal involvement, adjuvant chemotherapy is generally recommended. Currently, anthracycline-based schedules are preferred while in elderly patients, use of the CMF scheme has been described. However, adjuvant chemotherapy conferred a not statistically significant lower time to recurrence and improvement in overall survival [18].
No data exist about the adjuvant use of trastuzumab in MBC, and only one case report speculates about its efficacy in metastatic disease [22]. Considering that there is no biological reason for trastuzumab showing different activity in MBC than FBC patients, this treatment, and pertuzumab, might be considered for HER-2-positive MBC.
Tamoxifen remains the gold standard treatment for male patients. The 5-year OS is similar for tamoxifen-treated FBC and MBC patients (85.1% and 89.2% respectively; p = 0.972) [23]. Luteinizing hormone-releasing hormone (LHRH) agonists and orchiectomy are other available therapies. AI associated or not to LHRH agonist was evaluated in a retrospective series, reporting a response rate of 26.1% and a disease control rate of 56.5% [24][25]. In comparison with AI-treated FBC, MBC had a significantly poor outcome (5-year OS was 85.0% in FBC vs. 73.3% in MBC; p = 0.028) [23].
HT is indeed associated with a multitude of side effects in men. The most common adverse event related to tamoxifen is hot flashes. Decreased libido, weight gain, and malaise have been described. Less common are rash and erectile dysfunction. Alteration of hepatic function, lung embolism, thrombophlebitis, myalgia, depression, visual deficit, and diarrhea are uncommon [26]. Reported toxicities of AI use include decreased libido, leg swelling, and depression for anastrozole while edema and hot flashes have been reported after use of letrozole [27].
In metastatic MBC, chemotherapy has been offered as second- or third-line therapy after relapse of HT in ER-negative patients. Few and small studies highlight the role of chemotherapy in this setting of patients; the poor efficacy might be related to the old schedules used in those studies [28].
In the phase III OlympiAD trial germline BRCA1/2 metastatic BC patients were randomized to olaparib, a PARP inhibitor, versus a standard chemotherapy (capecitabine or eribulin, or vinorelbine). The study demonstrated a statistically significant advantage in median progression-free survival (mPFS) in patients treated with olaparib (mPFS = 7.0 months) vs patient treated with chemotherapy (mPFS = 4.2 months). Of note, about 2% of the enrolled population, respectively 5 out 205 patient in the olaparib group and 2 out of 97 in the contol group were male [29].
3. Cancer Genetic Counseling
In the clinical setting, professionals involved in the diagnosis and treatment of MBC patients should refer them to cancer genetic counseling services. Cancer genetic counseling should foresee risk assessment, adequate genetic testing, and an appropriate oncological preventive program for MBC patients and their at-risk healthy family members according to the specific genetic predisposition [30][31].
3.1. Risk Assessment
Genetic testing should be offered at diagnosis of breast cancer in a male patient, independently of a positive oncological family history [32]. However, the assessment of the ‘a priori’ probability of identifying a pathogenetic variant is an important component of pre-test counseling. Different probabilistic tools are available for risk assessment. Among these, one of the most widely validated is BRCAPRO [33][34]. Several studies have validated this model relative to FBC [35][36], but few data exist about MBC. In a recent Italian trial, Different prediction models were evaluated for their performance in 102 MBC patients with mutations in BRCA1/2 genes, diagnosed between 1991 and 2007. Thirty-nine out 102 patients (38%) of the patients reported a breast and ovarian cancer diagnosis in first- and/or second-degree relatives. Family history (FH) seems to be a better predictor of pathogenetic variants. Thus, pathogenetic variant carriers account for 15.4% (6/39) of MBC patients with a positive FH and 6.4% (4/63) of those without. Comparing different risk estimation models, only the BRCAPRO version 5.0 showed the best performance with a good sensitivity, specificity, and negative and positive predictive value [37]. More recently, BRCAPRO version 6.0 has been specifically validated as a counseling tool, showing a high performance in determining the ‘a priori’ risk of having a pathogenic variant of MBCs with or without positive FH [38].
The BOADICEA (Breast and Ovarian Analysis of Disease Incidence and Carrier Estimation Algorithm) program is an upcoming tool in European countries [39]. Since 2008, it has been updated and extended, considering the risks of MBC [40]. Previously, two studies compared BOADICEA, BRCAPRO, and other risk estimation models relative to MBC. However, they considered both FBC and MBC, the number of MBC cases evaluated was scarce, and inconsistent results were shown [41][42][43]. More recently, a comparison of BOADICEA, BRCAPRO, and the Myriad probabilistic models was performed in 307 male patients with BC and tested for BRCA1/2. Fifty-eight of these patients were BRCA1/2 carriers. BOADICEA was effective in predicting the total number of BRCA1/2 carriers, and there were non-significant differences in carriers-prediction performance between the BRCAPRO and the BOADICEA. Conversely, Myriad underestimated the number of carriers in almost 70% of the cases and therefore has not been considered a good carriers-predictive model for MBC patients [44].
3.2. Genetic Testing
Genetic testing should be offered within pre- and post-test counseling sessions according to the implications of the genetic test itself and the results [30][45][46]. Genetic testing for BRCA1/2 and PALB2 should be the first choice for mutational analysis. BRCA2 and PALB2 own similar clinical phenotype, i.e., a higher risk of solid tumors in children carriers of homozygotes pathogenetic variants. In addition, there is also an increase risk of developping BC in women. BRCA1/2 and PALB2 pathogenetic carriers in heterozygosis have also higher relative risk for pancreatic cancer [47][48][49]. BRCA2 and PALB2 share an analogue function and cancer predisposing spectrum; therefore, PALB2 genetic testing should be proposed to MBC patients belonging to families with BRCA1/2-negative or no informative genetic test results and to families in which a typical tumor of the BRCA2 spectrum are diagnosed. Other genes, i.e., TP53 and PTEN, should be considered if the family history is suggestive of minor syndromes, such as Li–Fraumeni and Cowden, respectively [32]. Moreover, mutational analysis of moderate-/low-penetrance genes should be considered if the genetic test is negative or not informative of pathogenetic variants in BRCA1/2. MBC patients should be counseled for the potential hereditary risk for relatives. Carriers of BRCA2 pathogenetic variants should be advised on the risk of Fanconi anemia and/or brain tumors in minor children if BRCA2 variants has been revealed in both maternal and paternal lines [50][51][52]. The most common pathogenetic variant situation would be in Iceland or in Ashkenazi Jews and no reports of Fanconi in double hits in Iceland or of c.5946delT have been described.
Figure 1 summarizes the genetic testing process for MBC.
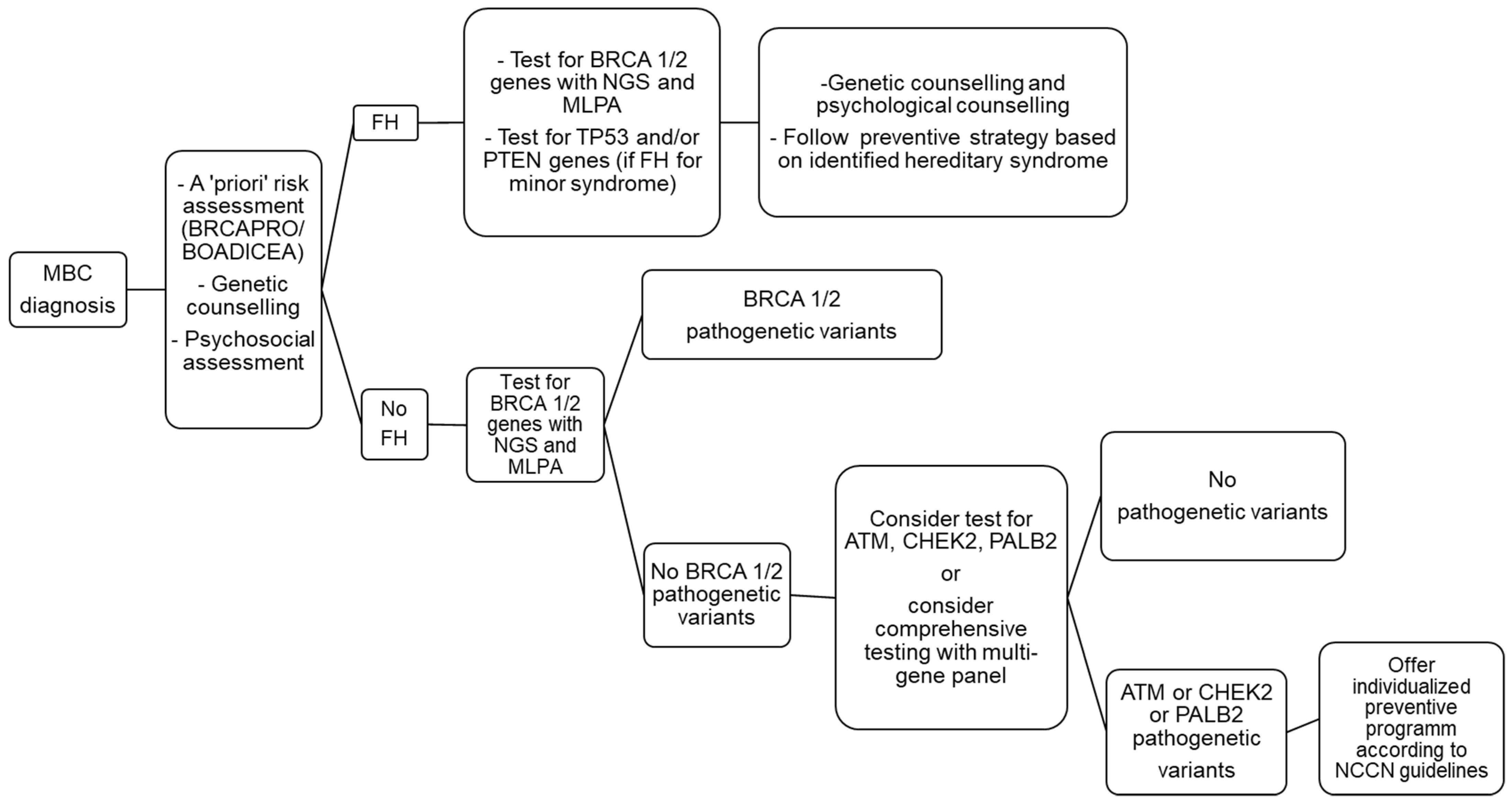
Figure 1. Genetic testing flow chart after a diagnosis of breast cancer (BC) in a male patient.
3.3. Management of Male Carriers of BRCA1/2 Pathogenetic Variants
BRCA status can impact on preventive options according to the cancer spectrum related to BRCA1 or BRCA2 pathogenetic variants. Moreover, the differential diagnosis of hereditary breast and/or ovarian cancer (HBOC) syndrome related to BRCA mutations and other minor syndromes, i.e., Li–Fraumeni and Cowden syndrome, on clinical basis and molecular definition by genetic testing can provide a new perspective in terms of prevention [32]. MBC patients have an increased risk of developing second primary tumors. In the SEER database review, that includes 4873 MBC cases, it has been reported a 1.9% incidence of second primary malignancy [53]. 21% of MBC patients received a diagnosis of a different primary tumor, such as prostate, colon, or genitourinary cancer.
BRCA pathogenetic variants are associated with higher risk of prostate cancer [15][54]. By panel testing, pancreatic cancer occurs in 0–3% for BRCA1 and 1–6% for BRCA2 [55][56][57][58].
Data on the differences in cancer risk association in male patients with BRCA1 and BRCA2 pathogenetic variants have emerged. Male BRCA2 carriers are more frequently affected by breast (OR, 5.47; 95% CI, 4.06–7.37; p < 0.001) and prostate (OR, 1.39; 95% CI, 1.09–1.78; p = 0.008) cancers and pancreatic cancers (OR, 3.00; 95% CI, 1.55–5.81; p = 0.001). Male BRCA2 carriers show an increased risk for developing two (OR, 7.97; 95% CI, 5.47–11.60; p < 0.001) and three (OR, 19.60; 95% CI, 4.64–82.89; p < 0.001) primary tumors [59][60]. In addition, Barnes et al. applied polygenic risk scores (PRS); which match specific SNPs associated to various diseases, to further stratify the class of risk in BRCA1/2 carrier patients. Silvestri et al. used the specific SNP breast and prostate cancer PRS to stratify male BRCA1 and BRCA2 carrier patients based on their absolute risk of developing BC and PC. This ultimately permitted the identification of two subgroups of male carriers, with the first having a higher risk of BC and PC and the second having low risk [61]. Taken together, all these results and their further validation could allow, in the near, future optimization of the screening surveillance of male BRCA1/2 carrier patients, offering an intensified and anticipated screening for high-risk carriers, and limiting and postponing prevention for low-risk carriers.
The National Comprehensive Cancer Network (NCCN) recommends the recruitment of all males carrying BRCA1 or BRCA2 pathogenetic variants within the same screening programs, especially for breast and prostate cancer [62]. More recently, the NCCN included melanoma in its screening algorithm in this subset [32] and recommend mammography only in males with gynecomastia or parenchymal/glandular breasts density [63].
This entry is adapted from the peer-reviewed paper 10.3390/cancers14082006
References
- Korde, L.A.; Zujewski, J.A.; Kamin, L.; Giordano, S.; Domchek, S.; Anderson, W.F.; Bartlett, J.M.; Gelmon, K.; Nahleh, Z.; Bergh, J.; et al. Multidisciplinary meeting on male breast cancer: Summary and research recommendations. J. Clin. Oncol. 2010, 28, 1114–1122.
- Doyle, S.; Steel, J.; Porter, G. Imaging male breast cancer. Clin. Radiol. 2011, 66, 1079–1085.
- Cutuli, B.; Le-Nir, C.C.; Serin, D.; Kirova, Y.; Gaci, Z.; Lemanski, C.; De Lafontan, B.; Zoubir, M.; Maingon, P.; Mignotte, H.; et al. Male breast cancer. Evolution of treatment and prognostic factors. Analysis of 489 cases. Crit. Rev. Oncol. Hematol. 2010, 73, 146–254.
- Chavez-MacGregor, M.; Clarke, C.A.; Lichtensztajn, D.; Hortobagyi, G.N.; Giordano, S.H. Male Breast Cancer According to Tumor Subtype and Race: A population-based study. Cancer 2013, 119, 1611–1617.
- Hultborn, R.; Hanson, C.; Köpf, I.; Verbiené, I.; Warnhammar, E.; Weimarck, A. Prevalence of Klinefelter’s syndrome in male breast cancer patients. Anticancer Res. 1997, 17, 4293–4297.
- Cardoso, F.; Bartlett, J.M.S.; Slaets, L.; van Deurzen, C.H.M.; van Leeuwen-Stok, E.; Porter, P.; Linderholm, B.; Hedenfalk, I.; Schröder, C.; Martens, J.; et al. Characterization of male breast cancer: Results of the EORTC 10085/TBCRC/BIG/NABCG International Male Breast Cancer Program. Ann. Oncol. 2017, 29, 405–417.
- Gargiulo, P.; Pensabene, M.; Milano, M.; Arpino, G.; Giuliano, M.; Forestieri, V.; Condello, C.; Lauria, R.; De Placido, S. Long-term survival and BRCA status in male breast cancer: A retrospective single-center analysis. BMC Cancer 2016, 16, 375.
- Humphries, M.P.; Sundara Rajan, S.; Honarpisheh, H.; Cserni, G.; Dent, J.; Fulford, L.; Jordan, L.B.; Jones, J.L.; Kanthan, R.; Litwiniuk, M.; et al. Characterisation of male breast cancer: A descriptive biomarker study from a large patient series. Sci. Rep. 2017, 7, 45293.
- Kornegoor, R.; Verschuur-Maes, A.H.; Buerger, H.; Hogenes, M.C.; de Bruin, P.C.; Oudejans, J.J.; Hinrichs, B.; van Diest, P.J. Immunophenotyping of male breast cancer. Histopathology 2012, 61, 1145–1155.
- Ottini, L.; Silvestri, V.; Rizzolo, P.; Falchetti, M.; Zanna, I.; Saieva, C.; Masala, G.; Bianchi, S.; Manoukian, S.; Barile, M.; et al. Clinical and pathologic characteristics of BRCA-positive and BRCA-negative male breast cancer patients: Results from a collaborative multicenter study in Italy. Breast Cancer Res. Treat. 2012, 134, 411–418.
- Cutuli, B. Strategies in treating male breast cancer. Expert Opin. Pharmacother. 2007, 8, 193–202.
- Sarmiento, S.; McColl, M.; Musavi, L.; Gani, F.; Canner, J.K.; Jacobs, L.; Fu, F.; Siotos, C.; Habibi, M. Male breast cancer: A closer look at patient and tumor characteristics and factors that affect survival using the National Cancer Database. Breast Cancer Res. Treat. 2020, 180, 171–479.
- Yadav, S.; Karam, D.; Bin Riaz, I.; Xie, H.; Durani, U.; Duma, N.; Giridhar, K.V.; Hieken, T.J.; Boughey, J.C.; Mutter, R.W.; et al. Male breast cancer in the United States: Treatment patterns and prognostic factors in the 21st century. Cancer 2020, 126, 16–36.
- Yadav, B.S.; Sharma, S.C.; Singh, R.; Dahiya, D.; Ghoshal, S. Male breast cancer: Outcome with adjuvant treatment. J. Cancer Res. Ther. 2020, 16, 1287–1293.
- Wernberg, J.A.; Yap, J.; Murekeyisoni, C.; Mashtare, T.; Wilding, G.E.; Kulkarni, S.A. Multiple primary tumors in men with breast cancer diagnoses: A SEER database review. J. Surg. Oncol. 2009, 99, 16–19.
- Gentilini, O.; Chagas, E.; Zurrida, S.; Intra, M.; De Cicco, C.; Gatti, G.; Silva, L.; Renne, G.; Cassano, E.; Veronesi, U. Sentinel lymph node biopsy in male patients with early breast cancer. Oncologist 2007, 12, 112–515.
- Flynn, L.W.; Park, J.; Patil, S.M.; Cody, H.S., 3rd; Port, E.R. Sentinel lymph node biopsy is successful and accurate in male breast carcinoma. J. Am. Coll. Surg. 2008, 206, 616–621.
- Yu, E.; Suzuki, H.; Younus, J.; Elfiki, T.; Stitt, L.; Yau, G.; Vujovic, O.; Perera, F.; Lock, M.; Tai, P. The impact of post-mastectomy radiation therapy on male breast cancer patients—A case series. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 196–700.
- Giordano, S.H.; Perkins, G.H.; Broglio, K.; Garcia, S.G.; Middleton, L.P.; Buzdar, A.U.; Hortobagyi, G.N. Adjuvant systemic therapy for male breast carcinoma. Cancer 2005, 104, 2359–2364.
- Volm, M.D. Male breast cancer. Curr. Treat. Options Oncol. 2003, 4, 159–164.
- Harlan, L.C.; Zujewski, J.A.; Goodman MT Stevens, J.L. Breast cancer in men in the United States: A population-based study of diagnosis, treatment, and survival. Cancer 2010, 116, 3558–3568.
- Eggemann, H.; Ignatov, A.; Smith, B.J.; Altmann, U.; von Minckwitz, G.; Röhl, F.W.; Jahn, M.; Costa, S.D. Adjuvant therapy with tamoxifen compared to aromatase inhibitors for 257 male breast cancer patients. Breast Cancer Res. Treat. 2013, 137, 465–470.
- Hayashi, H.; Kimura, M.; Yoshimoto, N.; Tsuzuki, M.; Tsunoda, N.; Fujita, T.; Yamashita, T.; Iwata, H. A case of HER2-positive male breast cancer with lung metastases showing a good response to trastuzumab and paclitaxel treatment. Breast Cancer 2009, 16, 136–140.
- Eggemann, H.; Altmann, U.; Costa, S.D.; Ignatov, A. Survival benefit of tamoxifen and aromatase inhibitor in male and female breast cancer. J. Cancer Res. Clin. Oncol. 2018, 144, 137–341.
- Doyen, J.; Italiano, A.; Largillier, R.; Ferrero, J.M.; Fontana, X.; Thyss, A. Aromatase inhibition in male breast cancer patients: Biological and clinical implications. Ann. Oncol. 2010, 21, 1243–1245.
- Zagouri, F.; Sergentanis, T.N.; Koutoulidis, V.; Sparber, C.; Steger, G.G.; Dubsky, P.; Zografos, G.C.; Psaltopoulou, T.; Gnant, M.; Dimopoulos, M.A.; et al. Aromatase inhibitors with or without gonadotropin-releasing hormone analogue in metastatic male breast cancer: A case series. Br. J. Cancer 2013, 108, 1259–1263.
- Pemmaraju, N.; Munsell, M.F.; Hortobagyi, G.N.; Giordano, S.H. Retrospective review of male breast cancer patients: Analysis of tamoxifen-related side-effects. Ann. Oncol. 2012, 23, 1471–1474.
- Visram, H.; Kanji, F.; Dent, S.F. Endocrine therapy for male breast cancer: Rate of toxicity and adherence. Curr. Oncol. 2010, 17, 17–21.
- Lopez, M.; Lauro, L.; Papaldo, P.; Lazzaro, B. Chemotherapy in metastatic male breast cancer. Oncology 1985, 42, 205–209.
- Robson, M.; Im, S.A.; Senkus, E.; Xu, B.; Domchek, S.M.; Masuda, N.; Delaloge, S.; Li, W.; Tung, N.; Armstrong, A.; et al. Olaparib for Metastatic Breast Cancer in Patients with a Germline BRCA Mutation. N. Engl. J. Med. 2017, 377, 123–533, Erratum in N. Engl. J. Med. 2017, 377, 1700.
- Trepanier, A.; Ahrens, M.; McKinnon, W.; Peters, J.; Stopfer, J.; Grumet, S.C.; Manley, S.; Culver, J.O.; Acton, R.; Larsen-Haidle, J.; et al. Genetic cancer risk assessment and counseling: Recommendations of the national society of genetic counselors. J. Genet. Couns. 2004, 13, 13–114.
- Contegiacomo, A.; Pensabene, M.; Capuano, I.; Tauchmanova, L.; Federico, M.; Turchetti, D.; Cortesi, L.; Marchetti, P.; Ricevuto, E.; Cianci, G.; et al. An oncologist-based model of cancer genetic counselling for hereditary breast and ovarian cancer. Ann. Oncol. 2004, 15, 726–732.
- Daly, M.B.; Pilarski, R.; Yurgelun, M.B.; Berry, M.P.; Buys, S.S.; Dickson, P.; Domchek, S.M.; Elkhanany, A.; Friedman, S.; Garber, J.E.; et al. NCCN Guidelines Insights: Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic, Version 1.2020. J. Natl. Compr. Cancer Netw. 2020, 18, 180–391.
- Berry, D.A.; Parmigiani, G.; Sanchez, J.; Schildkraut, J.; Winer, E. Probability of carrying a mutation of breast-ovarian cancer gene BRCA1 based on family history. J. Natl. Cancer Inst. 1997, 89, 227–238.
- Parmigiani, G.; Berry, D.; Aguilar, O. Determining carrier probabilities for breast cancer-susceptibility genes BRCA1 and BRCA2. Am. J. Hum. Genet. 1998, 62, 145–158.
- Marroni, F.; Aretini, P.; D’Andrea, E.; Caligo, M.A.; Cortesi, L.; Viel, A.; Ricevuto, E.; Montagna, M.; Cipollini, G.; Federico, M.; et al. Penetrance of breast and ovarian cancer in a large series of families tested for BRCA1/2 mutations. Eur. J. Hum. Genet. 2004, 12, 899–906.
- Euhus, D.M.; Smith, K.C.; Robinson, L.; Stucky, A.; Olopade, O.I.; Cummings, S.; Garber, J.E.; Chittenden, A.; Mills, G.B.; Rieger, P.; et al. Pretest prediction of BRCA1 or BRCA2 mutation by risk counselors and the computer model BRCAPRO. J. Natl. Cancer Inst. 2002, 94, 144–851.
- Zanna, I.; Rizzolo, P.; Sera, F.; Falchetti, M.; Aretini, P.; Giannini, G.; Masala, G.; Gulino, A.; Palli, D.; Ottini, L. The BRCAPRO 5.0 model is a useful tool in genetic counseling and clinical management of male breast cancer cases. Eur. J. Hum. Genet. 2010, 18, 156–858.
- Mitri, Z.I.; Jackson, M.; Garby, C.; Song, J.; Giordano, S.H.; Hortobágyi, G.N.; Singletary, C.N.; Hashmi, S.S.; Arun, B.K.; Litton, J.K. BRCAPRO 6.0 Model Validation in Male Patients Presenting for BRCA Testing. Oncologist 2015, 20, 193–597.
- Antoniou, A.C.; Pharoah, P.P.; Smith, P.; Easton, D.F. The BOADICEA model of genetic susceptibility to breast and ovarian cancer. Br. J. Cancer 2004, 191, 1580–1590.
- Antoniou, A.C.; Cunningham, A.P.; Peto, J.; Evans, D.G.; Lalloo, F.; Narod, S.A.; Risch, H.A.; Eyfjord, J.E.; Hopper, J.L.; Southey, M.C.; et al. The BOADICEA model of genetic susceptibility to breast and ovarian cancers: Updates and extensions. Br. J. Cancer 2008, 98, 1457–1466.
- Basham, V.M.; Lipscombe, J.M.; Ward, J.M.; Gayther, S.A.; Ponder, B.A.; Easton, D.F.; Pharoah, P.D. BRCA1 and BRCA2 mutations in a population-based study of male breast cancer. Breast Cancer Res. 2002, 4, 1.
- Panchal, S.M.; Ennis, M.; Canon, S.; Bordeleau, L.J. Selecting a BRCA risk assessment model for use in a familial cancer clinic. BMC Med. Genet. 2008, 9, 116.
- Kwong, A.; Wong, C.H.; Suen DT Co, M.; Kurian, A.W.; West, D.W.; Ford, J.M. Accuracy of BRCA1/2 mutation prediction models for different ethnicities and genders: Experience in a southern Chinese cohort. World J. Surg. 2012, 36, 702–713.
- Moghadasi, S.; Grundeken, V.; Janssen, L.A.M.; Dijkstra, N.H.; Rodríguez-Girondo, M.; van Zelst-Stams, W.A.G.; Oosterwijk, J.C.; Ausems, M.G.E.M.; Oldenburg, R.A.; Adank, M.A.; et al. Performance of BRCA1/2 mutation prediction models in male breast cancer patients. Clin. Genet. 2018, 93, 12–59.
- Riley, B.D.; Culver, J.O.; Skrzynia, C.; Senter, L.A.; Peters, J.A.; Costalas, J.W.; Callif-Daley, F.; Grumet, S.C.; Hunt, K.S.; Nagy, R.S.; et al. Essential elements of genetic cancer risk assessment, counseling, and testing: Updated recommendations of the National Society of Genetic Counselors. J. Genet. Couns. 2012, 21, 151–161.
- American Society of Clinical Oncology (ASCO). ASCO policy statement update: Genetic testing for cancer susceptibility. J. Clin. Oncol. 2003, 21, 1397–2406.
- Reid, S.; Schindler, D.; Hanenberg, H.; Barker, K.; Hanks, S.; Kalb, R.; Neveling, K.; Kelly, P.; Seal, S.; Freund, M.; et al. Biallelic mutations in PALB2 cause Fanconi anemia subtype FA-N and predispose to childhood cancer. Nat. Gen. 2007, 39, 162–164.
- Alter, B.P.; Rosenberg, P.S.; Brody, L.C. Clinical features associated with biallelic mutations in FANCD1/BRCA2. J. Med. Genet. 2007, 44, 1–9.
- Jones, S.; Hruban, R.H.; Kamiyama, M.; Borges, M.; Zhang, X.; Williams Parsons, D.; Cheng-Ho Lin, J.; Palmisano, E.; Brune, K.; Jaffee, E.M.; et al. Exomic sequencing identifies PALB2 as a pancreatic cancer susceptibility gene. Science 2009, 324, 21778.
- Burke, W.; Daly, M.; Garber, J.; Botkin, J.; Kahn, M.J.; Lynch, P.; McTiernan, A.; Offit, K.; Perlman, J.; Petersen, G.; et al. Recommendations for follow-up care of individuals with an inherited predisposition to cancer. II. BRCA1 and BRCA2. Cancer Genetics Studies Consortium. JAMA 1997, 277, 197–1003.
- Strahm, B.; Malkin, D. Hereditary cancer predisposition in children: Genetic basis and clinical implications. Int. J. Cancer 2006, 19, 2001–2006.
- Offit, K.; Levran, O.; Mullaney, B.; Mah, K.; Nafa, K.; Batish, S.D.; Diotti, R.; Schneider, H.; Deffenbaugh, A.; Scholl, T.; et al. Shared genetic susceptibility to breast cancer, brain tumors, and Fanconi anemia. J. Natl. Cancer Inst. 2003, 95, 1548–1551.
- Nicolosi, P.; Ledet, E.; Yang, S.; Michalski, S.; Freschi, B.; O’Leary, E.; Esplin, E.D.; Nussbaum, R.L.; Sarto, O. Prevalence of Germline Variants in Prostate Cancer and Implications for Current Genetic Testing Guidelines. JAMA Oncol. 2019, 5, 123–528.
- Giri, V.N.; Hegarty, S.E.; Hyatt, C.; O’Leary, E.; Garcia, J.; Knudsen, K.E.; Kelly, W.K.; Gomella, L.G. Germline genetic testing for inherited prostate cancer in practice: Implications for genetic testing, precision therapy, and cascade testing. Prostate 2019, 79, 133–339.
- Mandelker, D.; Zhang, L.; Kemel, Y.; Stadler, Z.K.; Joseph, V.; Zehir, A.; Pradhan, N.; Arnold, A.; Walsh, M.F.; Li, Y.; et al. Association Between Inherited Germline Mutations in Cancer Predisposition Genes and Risk of Pancreatic Cancer. JAMA 2018, 319, 1401–2409.
- van Asperen, C.J.; Brohet, R.M.; Meijers-Heijboer, E.J.; Hoogerbrugge, N.; Verhoef, S.; Vasen, H.F.A.; Ausems, M.G.E.M.; Menko, F.H.; Garcia, E.B.G.; Klijn, J.G.M.; et al. Cancer risks in BRCA2 families: Estimates for sites other than breast and ovary. J. Med. Genet. 2005, 42, 111–119.
- Moran, A.; O’Hara, C.; Khan, S.; Shack, L.; Woodward, E.; Maher, E.R.; Lalloo, F.; Evans, D.G.R. Risk of cancer other than breast or ovarian in individuals with BRCA1 and BRCA2 mutations. Fam. Cancer 2012, 11, 135–142.
- Silvestri, V.; Leslie, G.; Barnes, D.R.; CIMBA Group; Agnarsson, B.A.; Aittomäki, K.; Alducci, E.; Andrulis, I.L.; Barkardottir, R.B.; Barroso, A.; et al. Characterization of the Cancer Spectrum in Men with Germline BRCA1 and BRCA2 Pathogenic Variants: Results from the Consortium of Investigators of Modifiers of BRCA1/2 (CIMBA). JAMA Oncol. 2020, 6, 1218–1230, Erratum in JAMA Oncol. 2020, 6, 1815.
- Li, S.; Silvestri, V.; Leslie, G.; Rebbeck, T.R.; Neuhausen, S.L.; Hopper, J.L.; Nielsen, H.R.; Lee, A.; Yang, X.; McGuffog, L.; et al. Cancer Risks Associated with BRCA1 and BRCA2 Pathogenic Variants. J. Clin. Oncol. 2022.
- Barnes, D.R.; Silvestri, V.; Leslie, G.; McGuffog, L.; Dennis, J.; Yang, X.; Adlard, J.; Agnarsson, B.A.; Ahmed, M.; Aittomäki, K.; et al. Breast and Prostate Cancer Risks for Male BRCA1 and BRCA2 Pathogenic Variant Carriers Using Polygenic Risk Scores. J. Natl. Cancer Inst. 2022, 114, 109–122.
- Daly, M.B.; Axilbund, J.E.; Buys, S.; Crawford, B.; Farrell, C.D.; Friedman, S.; Garber, J.E.; Goorha, S.; Gruber, S.B.; Hampel, H.; et al. Genetic/familial high risk assessment: Breast and ovarian. J. Natl. Compr. Cancer Netw. 2010, 8, 162–594.
- Freedman, B.C.; Keto, J.; Rosenbaum Smith, S.M. Screening mammography in men with BRCA mutations: Is there a role? Breast J. 2012, 18, 13–75.
This entry is offline, you can click here to edit this entry!
