Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Developmental Biology
Neural tube defects (NTDs) are the second most common congenital malformations of humans, characterized by impaired development of the central nervous system. Even though the etiology of most birth defects remains undetermined, genetic and environmental risk factors in the background of NTDs have been identified and extensively reported.
- neural tube development
- neural tube defects
- neurogenesis
- spina bifida
- anencephaly
- neurulation
- congenital anomalies
1. Introduction
Congenital malformations entail single or multiple defects in the morphogenesis of organs that arise during embryogenesis and fetal development due to genetic mutations or maternal exposure to environmental factors, including infection. Together with neonatal disorders, congenital birth defects represent some of the leading causes of global disability-adjusted life years (DALYs) for both sexes, combined for all ages.
Primary congenital malformations arise from intrinsic errors within the developmental process and are of genetic origins. Those of secondary nature occur when environmental factors disrupt an otherwise normal developmental process during intrauterine life. These include any substances introduced into the organism (drugs, alcohol, smoking, etc.) or directly influencing exogenous factors (external temperature, environmental pollutants, etc.). These may cross the placenta and reach the fetus, inducing changes in the embryo–fetal development [1]. Estimates from the World Health Organization determine that 6% of infants worldwide present with congenital anomalies [2].
Neural tube defects are congenital malformations which arise during the abnormal embryonic development of the central nervous system. They include spina bifida, encephalocele and anencephaly, on top of many others. While spina bifida leads to impairment or loss of motor, sensory and autonomic function through damaging the spinal cord and nerves, encephalocele entails cerebral herniation, resulting in a variety of cognitive impairments [3]. On top of these functional impairments, most NTD patients also exhibit cosmetic concerns that might contribute to their morbidity as well as their psychological and financial conditions, greatly diminishing their wellbeing.
In order to combat the rising toll these congenital malformations have on infant survival and their quality of life, elaborate folic acid fortification programs have been introduced in many regions, including set requirements for food products such as wheat and maize flour [3]. In spite of elaborate folic acid fortification programs, many nations worldwide still experience disproportionately large numbers of NTDs and NTD-related deaths. This is largely due to the economic, health and social disparities present in those regions. As seen in the Global Burden of Disease data, the majority of deaths caused by NTDs (85%) occur in nations within the lowest GDP quartile [3], contributed to by limited access to neurosurgical care as well as inefficient prevention and education programs.
Therefore, even though we are witnessing a rise in improved diagnostic and therapeutic tools for prevention, management and treatment of congenital malformations, they still represent a major issue in the contemporary world.
2. Neural Tube Formation and Defects
2.1. Neural Tube Development
The third week of embryonic development in humans is marked with gastrulation, a process in which the three germ layers are formed: ectoderm, mesoderm and endoderm. The ectoderm further thickens in response to molecules which underly the notochord secretes, giving rise to the neural plate.
While the neural plate is the basis for primary neurulation, i.e., for the process of forming the neural tube from the neural plate, in amphibians, birds and mammals, the caudal portion of the neural tube develops directly by differentiation of the mesenchymal stem cells which represent the remnant of the primitive streak [4,5]. The process of the elevation and fusion of the opposing parts during formation of the (primary) neural tube is controlled by many signaling pathways, which can be, in general, described as either those coming from the notochord or those coming from the surrounding—for example, somatic—tissue. Signals coming from the notochord—such as Shh—have a strong ventralizing influence, i.e., they trigger many secondary genes which are then involved in the differentiation of the ventral specificity of the neural tube (e.g., OLIG2, NKX2-2). At the same time, the secretion of BMPs by the surface ectoderm and underlying mesoderm influences the differentiation of the dorsal portions in the closing of the neural tube.
Interestingly, the closure of the neural tube is species specific, and it differs in the number and position of sites which initiate the zippering. For example, while in birds, only two starting positions for the zippering exist (one cranial and one caudal), mammalians possess several closing regions in which closing occurs at the same time. While a pig possesses a huge region in the future thoracic portion of the body in which neural tissue first apposes and then fuses, humans possess at least four different regions in which zipper-like closing occurs at the same time [6]. Failure in the formation of the described processes results in different abnormalities called neural tube defects (NTDs) [7].
2.2. Neural Tube Defects (NTDs)
NTDs are one of the most common congenital malformations in humans. They form during the embryonic development and affect life quality from birth. The pathogenesis of NTDs has not yet been fully clarified, but nowadays, we know both genetic and environmental factors contribute to, or have an important role in, this malformation [8]. The presentation of NTDs is extremely variable, mostly depending on the localization of the lesion. Therefore, NTDs could be divided into open (Figure 1) and closed defects, depending on the exposure of the brain and/or spinal cord.
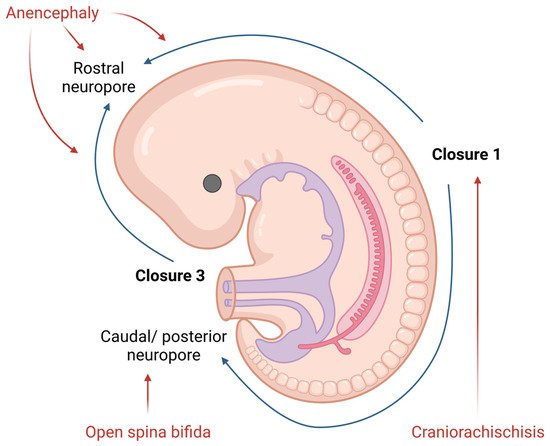
Figure 1. Diagram of neural tube closure and the origin of open NTDs in human embryos. Created with www.BioRender.com (accessed on 26 March 2022).
Open NTDs (ONTDs) such as craniorachischisis, anencephaly and myelomeningocele appear when there is a defect in the skull or vertebrae (Figure 1). Because the neural tissue is then exposed to the inherent toxicity of the amniotic fluid, this, consequentially, leads to the degradation. The diagnosis of such is usually set by an ultrasound. Despite an ultrasound, screening for the increase in the alpha-fetoprotein (AFP) concentration in the maternal serum became popular in NTD diagnostics as well [9].
The most severe defect, which includes both the cranial and spinal part of the neural tube, is craniorachischisis. This defect results in anencephaly and open spina bifida with an absence of the cranial vault and various defects of the vertebrae that can be seen on an ultrasound. Such condition is lethal, and no cure or surgical approach is known; therefore, the death of a newborn is unavoidable.
Anencephaly is a condition wherein the defect only affects the cranial part of the neural tube. Here, an absence of the forebrain and the skull can be noticed. Despite the anomalies of the calvarium [10], the facial bones appear normal. Furthermore, reported facial abnormalities include a flattened nasal bridge, low-set ears and eyes that seem to protrude [11]. Except for the aforementioned morphological features which could be seen on a routine ultrasound, the area of the cerebrovasculosa (residual cerebral tissue) and polyhydramnios (due to the lack of the swallowing reflex) could be detected. Like craniorachischisis, anencephaly also represents a lethal condition.
The failing posterior neural tube formation leads to myelomeningocele, a condition characterized by a protruded meningeal sack with neural placodes. However, when only the neural tissue is detectable, without the cystic sack, we talk about myelocele. Chiari malformation type II is usually associated with myelomeningocele and myelocele due to the traction of the brain stem throughout the opening in the vertebrae. The diagnosis of these is based on the detection of the “lemon” or “banana” indirect signs on a routine ultrasound checkup [12]. These NTDs present themselves as reduced leg movement in utero, due to the damage of the spinal cord, and various degrees of neurological impairment post birth. Even though these conditions entail debilitating impairments, newborn survival is high.
On the other hand, closed neural tube defects include the malformation of the fat, bone or membranes in the spinal column. The most common and powerful diagnostic tool used to detect these abnormalities is an ultrasound.
Encephalocele appears when a sack-like protrusion of the brain and/or meninges herniates through an opening in the skull. According to the tissue present in a herniated sack, cephaloceles could be classified as encephalomeningocystocele (herniated meninges, neural tissue and ventricles), encephalomeningocele (herniated meninges and neural tissue) and meningocele (herniated meninges). Depending on the location of the lesion, encephalocele are divided into anterior and occipital. Anterior encephalocele is subdivided into three more categories, frontal, sincipital and basal, while occipital is connected to Chiari malformation III. These abnormalities can be visualized using an ultrasound, where they present themselves as a sack protruding through a bone defect. Surgical correction of these is required after birth.
When compared with encephalocele, meningocele entails herniated meninges protruding through a vertebral arch defect, earning it the name of closed spine bifida. The herniated mass consisting of the dura and arachnoid is covered by skin which has atrophic epidermis without skin appendages. During the ultrasound examination, a cystic mass with spinal dysraphism is detected. The differences between meningocele and myelomeningocele are visible in cranial anatomy and the septate herniated mass observed in meningocele.
The aforementioned vertebral arch defect is also present in spina bifida occulta; however, no herniation or cystic masses are present. As such, the signs of spina bifida occulta are not as clear as the ones characterizing other NTDs. Therefore, it is important to look for cutaneous markers, including hair tufts, nevi, capillary hemangiomas, depigmentation regions or subcutaneous lipomas [13,14]. Because of this, spina bifida occulta is usually not recognized during pregnancy or in the perinatal period. Nevertheless, the most common type of spina bifida occulta with intradural lipoma could be detected in utero because lipomas and meningoceles have a similar appearance. The diagnosis of such NTDs is made easier in the postnatal period where a high-resolution ultrasound can be used. However, its sensitivity is affected by the presence of subcutaneous masses, impacting the diagnostic procedure quality. Even though the most precise diagnosis is made using magnetic resonance imaging, it is rarely used as the primary approach due to the high cost of this procedure. Because of this, the diagnosis of spina bifida occulta is usually made later in life because there is no disability present early on, and the first symptoms appear after the damage and/or traction of the cord.
This entry is adapted from the peer-reviewed paper 10.3390/biomedicines10050965
References
- Jasmina Isaković; Iva Šimunić; Denis Jagečić; Valentina Hribljan; Dinko Mitrečić; Overview of Neural Tube Defects: Gene–Environment Interactions, Preventative Approaches and Future Perspectives. Biomedicines 2022, 10, 965, 10.3390/biomedicines10050965.
This entry is offline, you can click here to edit this entry!
