You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Materials Science, Coatings & Films
The development of nanocomposite coatings is a rapidly growing field in the domain of nanotechnology. Nanocomposite coatings are rapidly being inducted in the sectors of aerospace, marine, automobiles, sensors, dental implants and electronics. Factors that affect the functionality of nanocomposite coatings include properties of matrices and fillers, spatial dispersion of fillers, surface morphology and deposition techniques.
- nanostructured coatings
- nanomaterials
- composite coatings
- corrosion
- wear
1. Polymer Matrix Nanocomposite Coatings
Polymer nanocomposite coatings which use polymers as matrices have received considerable interest in anticorrosion applications. By incorporating nanomaterial fillers in polymer matrices, improvement in several properties can be achieved, such as, stiffness, strength, corrosion resistance and wear resistance [116,117,118].
Nanostructured chitosan/ZnO coating was found to suppress corrosion on mild steel, with corrosion resistance improving as a function of increasing the number of layers of chitosan/ZnO [119]. Nanocomposite coating of oleic acid-modified chitosan/graphene oxide layer (CS/GO-OA) on a carbon steel substrate in NaCl solution increased corrosion resistance by 100-fold [116]. This improvement in corrosion resistance was ascribed to the decrease in hydrophilicity, oxygen permeability and ion transport because of the presence of the nanocomposite coating. Hydrophilicity of the nanocomposite coating was lowered because of the presence of a large alkyl group of oleic acid, whereas the formation of a barrier on the coating due to the interaction of functional groups between chitosan and oleic acid reduced ion transport through the nanocomposite coating. Graphene oxide reduced oxygen permeability [116].
The influence of graphene nanoplatelets (GNPs) on corrosion resistance of UHMWPE/GNPs nanocoatings deposited on AA2028 aluminium alloy substrate by electrostatic spraying was evaluated by comparing its corrosion resistance with that of the uncoated substrate and pure coating (pristine UHMWPE) [120]. Nanocoating with 2 wt % GNPs showed the maximum corrosion resistance, in 3.5% NaCl solution [120]. Mild steel coated with polyaniline coatings [121] containing 0% graphene (Pani) and PaniGn coatings containing 0.49, 1.92, 8.91 and 16.37 wt % graphene exhibited a significant corrosion reduction, by about 3–4 orders of magnitude, as compared to the uncoated mild steel. The nanocomposite coatings served as a physical barrier to the corrosive HCl environment while simultaneously imparting non-wetting properties. The coating with 1.92 wt % graphene provided the best corrosion resistance. Electrodeposited PaniGn nanocomposite coatings also improved the corrosion resistance of copper in 5000 ppm NaCl. The graphene-reinforced polyaniline coating generated a dense and compact layer, resulting in lower values of metal substrate corrosion potential and a lower rate of corrosion [122].
Anticorrosion performance can be greatly enhanced by incorporating treated nanoparticles in nanocomposite coatings. As an example, silicon dioxide (SiO2) nanoparticles were surface-treated with poly (styrene-co-butyl acrylate) to improve their dispersion in a fluoropolymer coating [123]. Enhanced corrosion resistance of the fluoropolymer nanocomposite coatings with treated silica nanoparticles up to a 4 wt % concentration of SiO2 was observed when coated on a steel substrate, compared to that of the uncoated steel substrate. However, the addition of SiO2 > 4 wt % weakened the link between the nanocomposite coating and the substrate, causing the nanoparticles to agglomerate, resulting in lower corrosion resistance [123].
2. Waterborne Polymer Nanocomposite Coatings
Volatile organic compounds (VOCs) are often used as plasticisers in paints to facilitate polymer dispersion and reduce ductility. However, the use of such substances is extremely detrimental to the environment [124,125]. A waterborne polymer coating, which uses water as a solvent instead of VOCs, was developed [126]. In comparison to the health risks and toxicity issues created by VOCs [125], waterborne polymer coatings provide advantages such as eco-friendliness, low viscosity, ease of cleaning, and non-toxicity [124]. Several researchers have investigated the corrosion behaviour of polymer-based waterborne coatings embedded with nanoparticles, such as Fe3O4, Fe2O3 and ZnO.
Waterborne epoxy acrylate-butylated melamine formaldehyde (EpAc-BMF) and ferrite (Fe3O4) nanocomposite coatings were examined for their corrosion performance (EpAc-BMF-Fe3O4) [124]. Corrosion resistance was tested in NaOH, NaCl and HCl solutions. EpAc-BMF-Fe3O4 nanocomposite coatings increased the corrosion resistance of mild steel samples in a salt spray test. An epoxy-based coating creates a protective barrier that prevents corrosive and aggressive ions from penetrating the steel surface [124]. The corrosion protection effect of colophony microcapsules incorporated in a waterborne acrylic coating, coated on a carbon steel substrate was examined [127]. It was observed that the addition of microcapsules improved corrosion resistance of the waterborne coatings. In two separate solutions with varying pH values, the microcapsule-doped coating maintained more noble Ecorr values and lower corrosion current density (Figure 8). Figure 9 shows SEM images of colophony microcapsules and coated steel specimens with and without doped microcapsules [127].
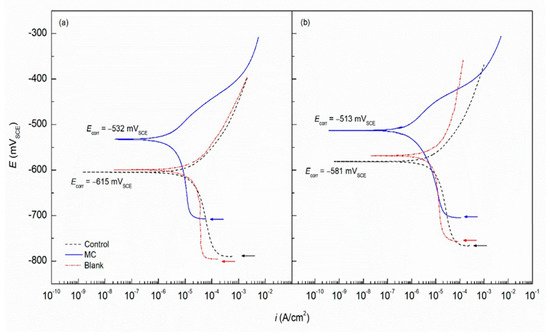
Figure 8. Potentiodynamic curves with acrylic coating doped with colophony microcapsules deposited on mild steel in (a) DI water (pH 6.8) and (b) SCPS (pH 12.6) [127].
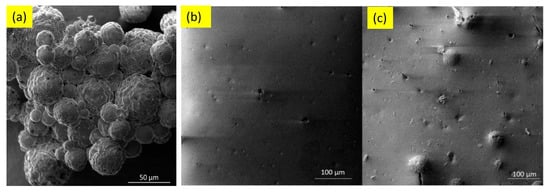
Figure 9. SEM images of (a) colophony microcapsules, (b) acrylic coating without microcapsules and (c) acrylic coating doped with microcapsules [127].
3. Metallic Matrix Nanocomposite Coatings
Reinforcements such as ceramic nanoparticles and carbon nanotubes that are inherently resistant to corrosion are incorporated in metallic matrices to produce nanocomposite coatings with increased corrosion resistance. Some examples are given here. The incorporation of SiC nanoparticles in Ni and Ni alloys resulted in the enhancement of corrosion resistance of the nanocomposite coatings [128,129]. Ni-P electroless coatings incorporated with SiC, Al2O3 and CeO2 nanoparticles increased their anticorrosion ability in NaCl and H2SO4 solutions. The addition of nanoparticles of SiO2 [130], Al2O3 [131,132,133] and CeO2 [134] to Ni-P electroless coatings improved the corrosion resistance of nanocomposite coatings in NaCl and H2SO4 solutions. The addition of carbon nanotubes (CNT) showed increased corrosion resistance of electroless Ni-P-CNT nanocomposite coatings in NaCl solution [135].
When compared to pure Zn coating, electrodeposited Zn-TiO2 nanocomposite coating performed better in (NH4)2SO4 solution with pH of 3 [136]. The best corrosion resistance was observed at 5 g/L nano-TiO2 concentration, due to inert oxide particles reducing the active surface in contact with the corrosive environment. The deterioration of corrosion performance at 10 g/L nanoparticle concentration was attributed to TiO2 nanoparticle aggregation and their non-uniform distribution [136].
This entry is adapted from the peer-reviewed paper 10.3390/nano12081323
This entry is offline, you can click here to edit this entry!
