Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Bisphenol A (BPA) is a synthetic compound utilized to manufacture plastics for Food Contact Materials (FCMs) or resins for the inside of food containers. Since it was recognized as an Endocrine-Disrupting Chemical (EDC), its implications in pathologies, such as cancer, obesity, diabetes, immune system alterations, and developmental and mental disorders, have been widely documented. Diet is considered the main source of exposure for humans to BPA. Consequently, continuous monitoring of the levels of BPA in foods is necessary to assess the risk associated with its consumption in one’s diet.
- bisphenols
- Endocrine-Disrupting Chemicals (EDCs)
- biosensors
- aptasensors
1. Introduction
Bisphenol A (BPA, Figure 1) is a synthetic chemical with a long history of use. Since 1950, it has been utilized to produce epoxy resins, and it has also been widely applied in the plastic industry as a monomer to synthesize polycarbonate polymers or as an additive in the synthesis of other kinds of plastics, such as polyvinyl chloride (PVC) [1]. BPA is mainly used in manufacturing Food Contact Materials (FCMs), such as storage containers, plastic water bottles, food packaging, and the inner coatings of food cans [2], but it is also contained in electronics equipment, children’s toys, dental sealants, thermal paper, and flame retardants.
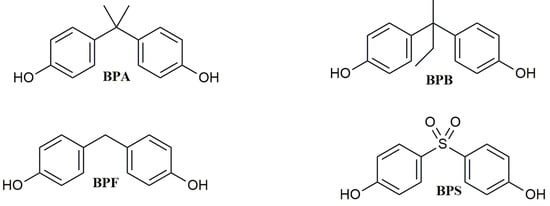
Figure 1. Chemical structure of BPA and its analogs.
2. BPA Aptamers: Sequence and Three-Dimensional Structure
Aptamers are sequence-specific single-stranded DNA or RNA oligonucleotides (12–200 nts) that are able to bind to a selected target with high affinity and selectivity. Many studies have shown that aptamers recognize their specific targets employing precise secondary or tertiary structures (duplex, G-quadruplex, stem-loop, etc.). Aptamers are selected by applying the Systematic Evolution of Ligands by EXponential enrichment (SELEX protocol) [41]. This procedure consists of an iterative selection and amplification process to identify, among a large pool of randomly generated single-stranded DNA/RNA sequences, the DNA/RNA molecules able to selectively bind to the immobilized target. After its development, SELEX protocol has been applied to obtain aptamers for different type of targets, varying from small molecules to proteins or also cells [42]. In analytical chemistry, aptamers have been successful applied as key elements to build up sensors able to capture and quali-quantitatively analyze a specific substance in a certain matrix. A general scheme of the principal steps required to build up an aptasensor is depicted in Figure 2.
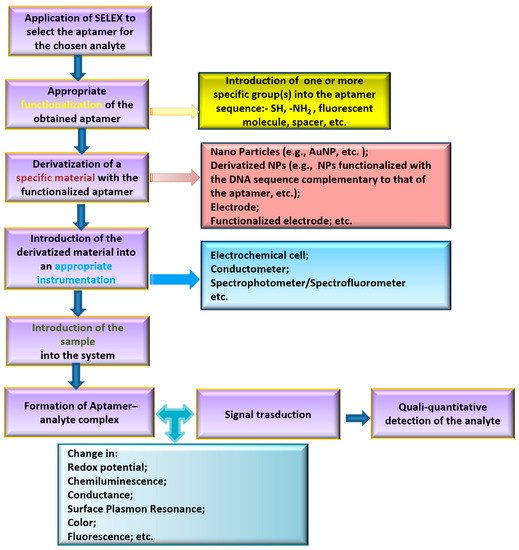
Figure 2. Scheme of the general strategy for aptasensor development.
Since the first anti-BPA aptamers were selected by Jo et al. [43], many efforts to develop sensitive BPA aptasensors for their use in different matrices have been made. With its high affinity and selectivity, BPA-Apt-2 (Table 1) is the main adopted sequence in the building up of aptasensors, and despite its 63 nt length, no truncated sequence has been developed until 2017 from the initial aptamer. Starting from the predicted secondary structure of the aptamer, Lee et al. [44] identified that BPA-Apt-3 (Figure 3 and Table 1) was able to bind to BPA with increased affinity and selectivity compared to BPA-Apt-2.
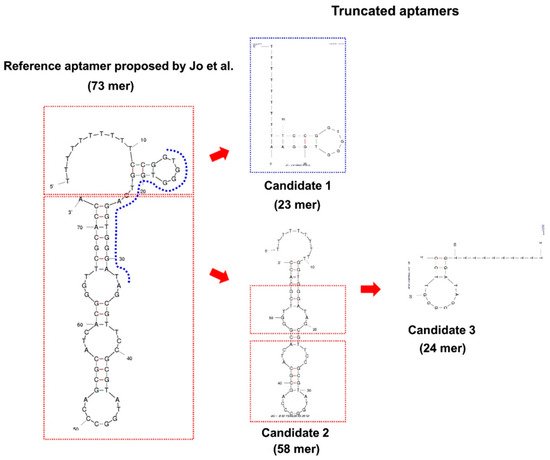
Figure 3. The 63 nts BPA-Apta-2 and the corresponding flow chart showing the process used to select the 24 nts truncated aptamer. Ten T residues at 5’-terminus of the initial sequence and of the corresponding truncated ones have been added. [Reprinted with permission from Lee, E.H.; Lim, H.J.; Lee, S.D.; Son, A. Highly Sensitive Detection of Bisphenol A by NanoAptamer Assay with Truncated Aptamer. ACS Appl. Mater. Interfaces 2017, 9, 14889–14898. Copyright 2017, ACS American Chemical Society].
Table 1. Aptamers against BPA used in biosensors for BPA detection and quantification in foods.
| Aptamer Binding BPA | Reference | ||||
|---|---|---|---|---|---|
| Acronym | Length | Sequence | Kd [nM] * | ||
| BPA-Apt-1 | 60 nts | CCGCCGTTGGTGTGGTGGGCCTAGGGCCGGCGGCGCACAGCTGTTATAGACGCCTCCAGC | not reported | [43] | Table 1, group 2; ID #6 * |
| BPA-Apt-2 | 63 nts | CCGGTGGGTGGTCAGGTGGGATAGCGTTCCGCGTATGGCCCAGCGCATCACGGGTTCGCACCA | 8.3 ** | [43] | Table 1, group 7; ID #3 * |
| BPA-Apt-3 | 24 nts | TTTTTTTTTTGGATAGCGGGTTCC | not reported | [44] | Truncated BPA-Apt-2 |
| BPA-Apt-4 | 38 nts | TGGGTGGTCAGGTGGGATAGCGTTCCGCGTATGGCCCA | 13.17 ± 1.02 *** | [45] | Truncated BPA-Apt-2 |
| BPA-Apt-5 | 12 nts | GGATAGCGTTCC | 27.05 ± 2.08 *** | [45] | Truncated BPA-Apt-2 |
| BPA-Apt-6 | 23 nts | TTTTTTTTTTCCGGTGGGTGGAA | 1190.61 ± 66.05 *** | [44] | Truncated BPA-Apt-2 |
In 2020, a similar truncation protocol was adopted by Jia et al. [45], succeeding in obtaining two differently truncated sequences (Figure 4).
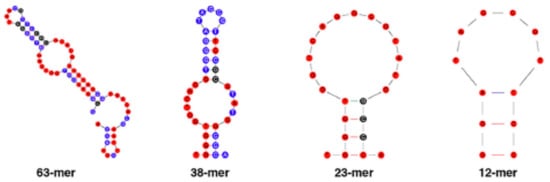
Figure 4. The flow chart from BPA-Apt-2 to the two selected 38 and 12 nts truncated aptamers [45]. [Reprinted from Food Chem, 317, Jia, M.; Sha, J.; Li, Z.; Wang, W.; Zhang, H. High affinity truncated aptamers for ultra-sensitive colorimetric detection of bisphenol A with label-free aptasensor 126459–12467. Copyright 2020, with permission from Elsevier].
The comparison between the three-dimensional structure predicted for BPA-Apt-3 and that of BPA-Apt-5 shows the presence of the same stem, formed by the 5’-GGA and 3’-TCC, and a differently long loop containing a common AGCGT sequence in both the folded aptamers. These two works confirm that SELEX alone is unsatisfactory for obtaining an efficient and cost-effective aptamer. Therefore, further studies are necessary to identify the exact binding mode of BPA to its aptamers and/or to the shortened aptamer derivatives as well as the secondary structures adopted in solution by the aptamer(s) alone and in the BPA–aptamer complex(es). Indeed, only a few studies reported the molecular modeling of aptamers and their BPA complexes [44,45], whereas in most cases, the stem-loop aptamer structures have been derived based on predictive DNA programs, and only in few cases they were confirmed by circular dichroism analysis. Notably, some authors mentioned the ability of BPA to induce the folding of BPA-Apt-2 (Table 1) into G-quadruplex(s) [47,48,49,50], although this hypothesis requires further experimental evidence.
3. Aptamer-Based Biosensor Applications Tested for the Determination of BPA in Real Food Samples
So far, aptamer-based biosensors have not been applied to an extensive monitoring of the levels of BPA in food samples. However, several authors reported the designs of various kinds of aptasensors to be specifically used in the detection of BPA in food samples. After their construction, all the aptasensors were tested to verify their feasibility in the analysis of real samples. All foods used in the applicability tests were liquids, mostly tap or mineralized water [45,49,51,52,53,54,55,56,57,58,59,60,61], followed by milk and fruit juices [47,53,59,60,62,63] and red wine samples [64]. Either no treatment (water samples) or just a quick pre-treatment of samples was necessary. Fruit juices were filtered to remove any residues of solid particles. Milk samples required a slightly time-consuming pre-treatment to eliminate fats by centrifugation and to eliminate proteins by precipitation and further centrifugation. Mirzajani et al. [65] analyzed two different brands of canned food (green beans and sweet peas), but they carried out the experiments only on the liquid portion of the cans. However, studies in the literature demonstrated that, when canned foods are analyzed, the BPA concentrations are higher in the solid portion than those detected in the liquid portion [33,66]. Especially in the case of canned seafoods, this should be considered in relation to the high probability of contamination due to the ingestion of microplastics by fish in the marine environment [6,8,9]. At last, Lee et al. [67] investigated the application of their colorimetric aptasensor directly on grains of commercially available steamed rice pre-treated with a solution of BPA.
The limit of detection (LOD) and recovery at different concentrations of BPA were the main performance parameters that the authors evaluated. In addition, the selectivity of each aptasensor was generally assessed vs. possible interferent substances or BPA analogs, including some compounds currently used as substitutes of BPA in plastic manufacturing. In several studies the reproducibility was also measured. Both selectivity and reproducibility proved to be The LODs, estimated mostly using BPA solutions at different concentrations, were generally very low. Jia et al. [45] compared the performance of their aptasensor to other more traditional methods. They showed that the LOD obtained by the aptasensor was generally lower, except for the case of GC–MS and electrochemical methods. Nonetheless, the authors concluded that their detection technique was preferable considering the undoubted advantages offered by the aptasensor, such as a low cost and a simple detection process in addition to a still-high sensitivity.
Generally, aptasensors tested in food samples showed good stability. Only Ye et al. found that their biosensor was not very stable when compared to the LC–MS/MS method, although they suggested that this problem could be overcome by carefully checking the temperature and buffering solution [59].
This entry is adapted from the peer-reviewed paper 10.3390/app12083752
This entry is offline, you can click here to edit this entry!
