Pharmacological profile of phytochemicals has attracted much attention to their use in disease therapy. Since cancer is a major problem for public health with high mortality and morbidity worldwide, experiments have focused on revealing the anti-tumor activity of natural products. Flavonoids comprise a large family of natural products with different categories. Chrysin is a
hydroxylated flavonoid belonging to the flavone category. Chrysin has demonstrated great potential in treating different disorders, due to possessing biological and therapeutic activities, such as antioxidant, anti-inflammatory, hepatoprotective, neuroprotective, etc. Over recent years, the anti-tumor activity of chrysin has been investigated, and in the present review, we provide a mechanistic discussion of the inhibitory effect of chrysin on proliferation and invasion of different cancer cells. Molecular pathways, such as Notch1, microRNAs, signal transducer and activator of transcription 3 (STAT3), nuclear factor-kappaB (NF-κB), PI3K/Akt, MAPK, etc., as targets of chrysin are discussed. The efficiency of chrysin in promoting anti-tumor activity of chemotherapeutic agents and suppressing drug resistance is described. Moreover, poor bioavailability, as one of the drawbacks of chrysin, is improved using various nanocarriers, such as micelles, polymeric nanoparticles, etc. This updated review will provide a direction for further studies in evaluating the anti-tumor activity of chrysin.
- chrysin
- cancer therapy
- nanoparticle
- flavonoid
- chemotherapy
1. Introduction
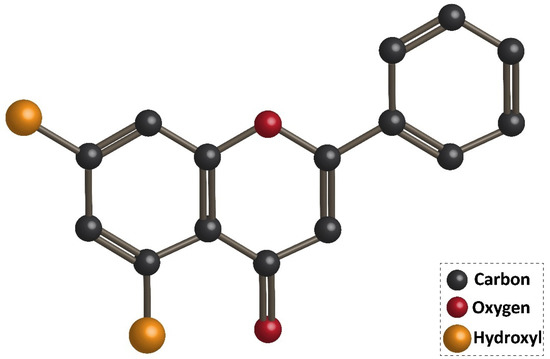
Flavonoids are the largest group of plant secondary metabolites with favorable health-promoting effects [46,47,48,49]. The interest in flavonoids has been increased, since these valuable compounds act through various physiological mechanisms and affect a wide variety of signaling networks. Dietary intake of flavonoids is estimated to be 50 and 800 mg per day [50,51]. Chrysin is a hydroxylated flavonoid belonging to flavone class, and is extensively found in sources, such as honey, propolis, and plant species [52,53]. Noteworthy, chrysin occurs in natural sources with different concentrations. For instance, the concentration of chrysin in honeydew honey is 0.10 mg/kg, while it has a higher concentration (5.3 mg/kg) in forest honeys [54]. The content of chrysin in propolis is estimated to be 25 g/L [55]. Chrysin concentration in mushrooms is at the range of 0.17–0.34 mg/kg [56]. The IUPAC name of chrysin is 5,7-dihydroxy-2-phenyl-4H-chromen4-one and 5,7-dihydroxyflavone. Figure 1 demonstrates the chemical structure of chrysin. The chrysin structure has similarities and differences with the flavonoid family. Structurally, chrysin has two benzene rings (A and B) with one oxygen consisting of a heterocyclic ring. Chrysin lacks a 3-carbon hydroxyl group, but it has 2–3 double-bound carbon with a carbonyl group attached to 4th carbon. The chemical structure of chrysin demonstrates that it has –OH group at 5th and 7th carbon atoms. There is a difference in the structure of chrysin and other flavonoids, so that chrysin does not possess any oxygenation in ring B (Figure 1). It has been reported that changes in ring A of chrysin account for the generation of different derivatives of chrysin, such as wogonin, baicalein, and oroxylin [57].
Accumulating data demonstrates that poor absorption, rapid metabolism, and systemic elimination are responsible for poor bioavailability of chrysin in humans that, subsequently, restrict its therapeutic effects [58]. It is worth mentioning that oxidation in intestinal and hepatic cells is not responsible for the metabolism of chrysin in the body. In contrast, conjugation pathways, such as glucuronidation and sulfation catalyze chrysin. Enzymes, such as P-PST, M-PST, and UGT1A6, contribute to the metabolism of chrysin, and their high affinity for chrysin can justify the poor bioavailability of this natural compound. Clinical studies have shown that the plasma concentration of chrysin following oral administration is very low [59]. Notably, serum concentrations of chrysin have not been reported yet, but it can be predicted based on other flavonoids. Since flavonoid aglycones demonstrate serum concentration as low as 1 µmol/L [60], the serum concentration of chrysin would be at the range of nanomolar. The studies related to the absorption of chrysin demonstrate that its sulfation and glucuronidation limit the absorption of this valuable compound in the intestine. MRP2 transporters are involved in the efflux of chrysin metabolites from the intestine, and in the lumen, sulfatases and glucuronidases hydrolyze metabolites into chrysin. This leads to the emergence of chrysin in stool, but high contents of chrysin in stool demonstrates that it has low absorption [61]. Some strategies have been applied in promoting bioavailability and absorption of chrysin, such as using nanoscale delivery systems [62].
2. Chrysin and Its Pharmacological Activities
In previous sections, we provided explanations about the role of natural products in cancer therapy, and then, we introduced the chemistry and pharmacokinetics of chrysin. In this section, we aim to describe the pharmacological activities of chrysin, based on the newly published article—which is summarized in Table 1.
Table 1. Various pharmacological activities of chrysin in treating diseases.
| Therapeutic Effect/Disease | In Vitro/In Vivo | Cell Line/Animal Model | Dose (In Vivo)/Concentration (In Vitro) | Duration of Experiment | Administration Route | Outcomes | Refs |
|---|---|---|---|---|---|---|---|
| Anti-hypertension | In vivo | Rat | 100 mg/kg | 18 weeks | Oral administration | Decreasing systolic and diastolic pressures Reducing insulin, angiotensin II and tiacylglycerols levels |
[85] |
| Neuroprotective | In vivo | Rat | 10 and 30 mg/kg | 8 weeks | Oral gavage | Improving memory impairment Enhancing neuronal cell survival Reducing hippocampal neurogenesis depletion |
[86] |
| Neuroprotective | In vivo | Rat | 10, 30, and 100 mg/kg | 3 weeks | Oral administration | Enhancing GPx activity and number of surviving cells in hippocampus Reducing MDA, NO and PGE2 levels Improving passive avoidance memory |
[87] |
| Cardioprotective | In vitro | Cardiomyocyte | 10, 50, and 100 µM | 3 h | - | Decreasing aluminium-phosphide-mediated oxidative stress Reducing mitochondrial damage Improving mitochondrial function |
[88] |
| Renoprotective Hepatoprotective |
In vivo | Rat | 100 mg/kg | - | - | Reinforcing antioxidant defense system via up-regulating GSH and SOD activities Reducing lipid peroxidation Decreasing inflammation via TNF-α down-regulation |
[89] |
| Renoprotective Hepatoprotective |
In vivo | Rat | 25 and 50 mg/kg | 7 days | Oral administration | Reducing AST, ALT, ALP, urea, creatinine, MDA and hepatorenal deterioration Enhancing SOD, CAT, and GPx activities Apoptosis inhibition via Bcl-2 up-regulation and Bax down-regulation Reducing inflammation via NF-κB down-regulation |
[90] |
| Anti-diabetic | In vitro | Chorioretinal endothelial cells | 1, 3, 10, 30, and 50 µM | 24 h | - | Reducing Akt, ERK, MMP-2, and VEGF expressions | [91] |
| Anti-diabetic | In vivo | Rat model of type I diabetes | 50 and 100 mg/kg | 28 days | Oral gavage | Reducing oxidative stress index Enhancing glutathione levels |
[92] |
| Gastric healing | In vivo | Mouse model of gastric ulcer via ethanol | 10, 50, and 100 mg/kg | 7 and 14 days | Oral administration | Apoptosis inhibition via caspase-3 down-regulation Reducing macroscopic lesions Enhancing catalase activity Improving inflammation via COX-2 down-regulation |
Increasing evidence demonstrates that chrysin possesses health-promoting effects, including antioxidant [63,64], anti-inflammatory [65], anti-diabetes [66], neuroprotective [67], hepatoprotective [68], cardioprotective [69], lipid-lowering effect [70], etc. These therapeutic effects have made chrysin as a suitable option in disease therapy. Non-alcoholic fatty liver disease (NAFLD) is one of the most common metabolic disorders, and to date, natural products have shown great potential in the alleviation of NAFLD. Similarly, a recently recorded article has revealed that chrysin administration (25, 50, and 100 mg/kg) alleviates NAFLD in rats via reducing serum fasting glucose that subsequently improves insulin resistance and dyslipidemia. Noteworthy, chrysin can significantly diminish liver weight by reducing hepatic free fatty acids, triglyceride, and cholesterol content. Anti-inflammatory and antioxidant activities of chrysin are also involved in the amelioration of NAFLD via decreasing lobular inflammation, steatosis, and carbonyl content [71]. Many reports demonstrate that chrysin can be beneficial in reducing acetaminophen-mediated hepatotoxicity in rats. In this regard, chrysin reduces levels of pro-inflammatory cytokines, such as tumor necrosis factor-α (TNF-α) and interleukin-2 (IL-2). The ameliorative effect of chrysin on acetaminophen-mediated hepatotoxicity seems to be dose-dependent with more therapeutic effects at higher concentrations [72]. In addition to hepatoprotective activity, chrysin has shown potential neuroprotective effects. One of the complications causing neuronal cell death is ischemic-reperfusion (I/R) injury. Inflammation and oxidative stress are two main mechanisms involved in I/R injury [73,74,75]. Chrysin administration (10 and 20 mg/kg) reduces pro-inflammatory factors (TNF-α, IL-1β, and IL-6) and oxidative stress to alleviate cerebral I/R injury. Investigation of molecular pathways reveals that the induction of the PI3K/Akt signaling pathway by chrysin contributes to a reduction in oxidative stress and inflammation during cerebral I/R injury [76]. The inhibitory effect of chrysin on inflammation and oxidative stress is also important in Parkinson’s disease (PD) treatment [77]. Chrysin (25, 50, and 100 mg/kg) improves cognitive capacity, inflammation, and apoptosis to ameliorate traumatic brain injury (TBI) [78]. Overall, the literature confirms the health-promoting and therapeutic effects of chrysin that are important in disease therapy, and the effect of this valuable compound on molecular pathways (Figure 2) [79,80,81,82]. In the next sections, we specifically discuss the role of chrysin in cancer therapy [83,84].
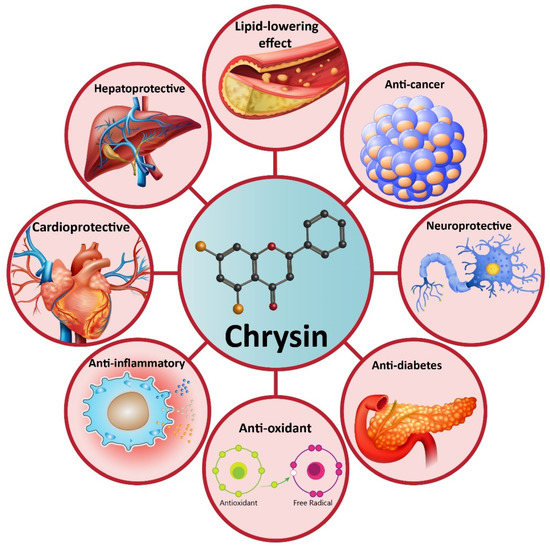
Figure 2. A schematic representation of the health-promoting effects of chrysin in pre-clinical experiments.
3. Chrysin-Loaded Nanoparticles in Cancer Therapy
Micelles have attracted much attention in cancer therapy, due to their potential to deliver anti-tumor agents [288,289]. Self-assembled micelles are amphiphilic copolymers with size at the range of 10–100 nm. Micelles have high cellular uptake and passive targeting functions to tumor known as enhanced permeability [290,291]. Recently, chrysin- and docetaxel-loaded micelles have been applied in enhancing the efficacy of chemotherapy. This co-delivery by micelles exerts a synergistic effect on chemotherapy and effectively suppresses migration and invasion of cancer stem cells. Chrysin- and docetaxel-loaded micelles enhance levels of ROS to impair cancer stem cell viability. Notably, enhanced the anti-tumor activity of chrysin and docetaxel against cancer cells is due to their enhanced accumulation in cancer cells by micelles [292]. Polymeric micelles have also been designed in co-delivery of chrysin and methotrexate in the chemotherapy of breast cancer cells. The idea of using a chemotherapeutic agent with a natural anti-tumor agent is that this combination is important in sensitizing cancer cells into chemotherapy. Using nanoparticles promotes cytotoxicity against cancer cells via enhancing cellular uptake. Based on the small size of polymeric micelles (around 55 nm), they can escape from macrophages and kidney filtration to reach into the tumor site, providing targeted delivery of anti-tumor compounds [293].
Another study has applied polyurea dendrimers for delivery of chrysin in ovarian cancer therapy. Polyurea dendrimers are three-dimensional polymers with urea moieties in the backbone and peripheral amine groups. They possess various beneficial properties, including water-solubility, biocompatibility, biodegradability, and pH-sensitivity, making them suitable options in drug delivery [294]. Furthermore, as cancer cells overexpress folate receptors on their surface [295,296], surface functionalization of nanoparticles with folate can be advantageous in enhancing cellular uptake of these nanoparticles and providing selective targeting. Chrysin- and selenium-loaded dendrimers are capable of induction of oxidative stress and reducing the viability of OC cells. Furthermore, they demonstrate no toxicity against normal cells that can be attributed to using folate for the functionalization of dendrimers [297].
Polymeric nanoparticles possess a core-shell structure that self-assemble in an aqueous medium. The hydrophilic shell is responsible for preserving the stability of nanoparticle, and the hydrophobic core encapsulates anti-tumor drug. Synthetic polymers, including poly (e-caprolactone) (PCL), polyglycolide (PGA), and polylactides (PLA), are applied in biomedical applications, due to their features, such as biocompatibility, high permeability, predictable degradation kinetics, etc., that are important in the field of biomedicine [298,299,300]. However, crystallinity and low biodegradation are drawbacks of PCL that can be solved using monomers. Poly (ethylene glycol) (PEG) is a safe, flexible, and hydrophilic agent approved by the Food and Drug Administration (FDA) that can be used internally in the human body [298,301,302,303]. Chrysin-loaded polymeric nanoparticles have been applied in breast cancer therapy. The results demonstrate that targeted delivery of chrysin at the tumor site by polymeric nanoparticles leads to enhanced anti-tumor activity, due to enhanced cellular uptake [304].
Nanoparticles can provide a platform for co-loading of chrysin with other natural anti-tumor compounds, such as curcumin. Briefly, curcumin is isolated from the rhizome of curcuma longa and has potent anti-tumor activity against different cancer cells [305]. Using nanoparticles can significantly enhance the bioavailability and therapeutic effects of curcumin [306]. Curcumin- and chrysin-loaded PLGA-PEG nanoparticles have been designed in CRC therapy. This co-loading exerts a synergistic effect and enhances the cytotoxicity of these phytochemicals against CRC cells [307]. Studies demonstrate that telomerase activity is associated with enhanced proliferation and invasion of cancer cells. Catalytic domain (hTERT) participates in telomerase gene overexpression that has been reported in CRC [308,309]. Chrysin- and curcumin-loaded nanoparticles effectively down-regulate the expression of hTERT in suppressing the progression of CRC cells [307]. In addition to the anti-proliferative activity via hTERT down-regulation, chrysin- and curcumin-loaded nanoparticles can suppress metastasis of cancer cells via reducing expressions of MMP-2 and MMP-9 [310].
Several homologous proteins known as tissue inhibitors of metalloproteinase (TIMPs) can regulate the activity of MMPs. TIMP-1 and TIMP-2 are capable of reducing the expression of MMP-2 and MMP-9 in suppressing metastasis and migration of cancer cells [311]. Chrysin- and curcumin-loaded nanoparticles significantly promote the expression of TIMP-1 and TIMP-2 to exert a reduction in melanoma invasion [310]. Taking everything into account, studies agree with the fact that nanoparticles can enhance the anti-tumor activity of chrysin against cancer cells [62,312,313,314,315,316]. Nanoparticles can provide a platform for the co-delivery of chrysin and other anti-tumor agents that is important in promoting its inhibitory effect against cancer cells (Figure 4) (Table 3). Further studies can focus on developing other types of nanocarriers, such as carbon nanotubes, liposomes, etc., for delivery of chrysin in cancer therapy.
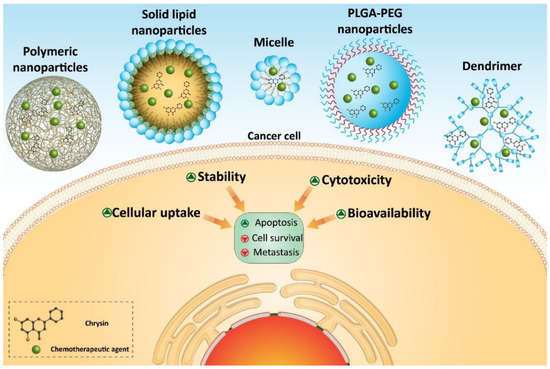
Table 3. Chrysin-loaded nanoparticles in cancer therapy.
| Nanovehicle | Cancer Type | In Vitro/In Vivo | Cell Line/Animal Model | Particle Size (nm) | Zeta Potential (mV) | Encapsulation Efficiency (%) | Outcomes | Refs |
|---|---|---|---|---|---|---|---|---|
| Micelle | Colorectal cancer | In vitro | Human-derived epithelial colorectal cancer cell lines HT-29 | 72–142 | +10.1 | 77 (Docetaxel) 44 (chrysin) |
Enhanced cellular uptake Effective inhibition of cancer stem cell migration |
[292] |
| Polymeric micelles | Breast cancer | In vitro | MCF-7 cells | 55 | −2.7 | 87.6 (methotrexate) 86.5 (chrysin) |
Enhancing efficacy of chrysin and methotrexate in breast cancer therapy via promoting cellular uptake | [293] |
| Dendrimer | Ovarian cancer | In vitro | Serous carcinoma (OSC) cell lines (OVCAR3 HTB-161™ and OVCAR8 CVCL_1629™) and a clear cell carcinoma (OCCC) cell line (ES2 CRL-1978™) | - | - | - | Selective targeting of cancer cells by folate functionalization of dendrimers High cellular uptake Remarkable decrease in survival of cancer cells |
[297] |
| Polymeric nanoparticles | Breast cancer | In vitro | T47D breast cancer cell line | 75 | - | 99.89 | Higher cytotoxicity against breast cancer cells compared to chrysin alone | [304] |
| PLGA-PEG nanoparticles | Breast cancer | In vitro | T47-D breast cancer cell line | 20–75 | - | 70 | High cytotoxicity Excellent cellular uptake and encapsulation efficiency |
[317] |
| PLGA-PEG nanoparticles | Colorectal cancer | In vitro | SW480 cells | 50–140 nm | - | Higher cytotoxicity compared to chrysin and curcumin alone hTERT down-regulation |
[307] | |
| PLGA-PEG nanoparticles | Melanoma | In vivo | C57B16 mice bearing B16F10 melanoma tumours | 285 | −3.7 | 78.27 (curcumin) 83.5 (chrysin) |
Enhancing expression of TIMP-1 and TIMP-2 Down-regulation of MMP-2 and MMP-9 Suppressing metastasis of cancer cells |
[310] |
| Solid lipid nanoparticles | Breast cancer | In vitro | MCF-7 cells | Below 500 | −20 to −47 | More than 90% | High stability and promoting the anti-tumor activity of chrysin | [312] |
| PLGA-PEG nanoparticles | Breast cancer | In vitro | T47D cells | 70–300 | - | 99.89 | Accumulation in breast cancer cells High cytotoxicity |
[318] |
| PLGA-PEG nanoparticles | Breast cancer | In vitro | MDA-MB-231 cells | 305 | −3.8 | 80.22 (curcumin) 85.25 (chrysin) |
Synergistic effect Cell cycle arrest at G2/M phase Apoptosis induction Up-regulation of miR-132 and miR-502c |
[319] |
| Copolymer nanoparticle | Lung cancer | In vitro In vivo |
A549 cells Mice bearing an A549-derived tumor |
77 | −2.22 | 46.96 | Enhanced cytotoxicity More potential in exerting tumor growth delay |
[320] |
| Micelle | Breast cancer | In vitro | MCF-7 cells | 152–420 | −21.6 | 52–89 | Promoting bioavailability of chrysin Exerting a 5-fold increase in anti-tumor activity |
[321] |
| PLGA-PEG nanoparticles | Gastric cancer | In vitro | AGS cells | 70–300 | - | 98.6 | Decreasing cell survival via down-regulation of miR-18a, miR-21, and miR-221 | [322] |
4. Conclusions and Remarks
Chrysin affects various molecular pathways and mechanisms in cancer therapy. Apoptosis is the most well-known target of chrysin in cancer therapy, and both intrinsic and extrinsic pathways of apoptosis are induced by chrysin in cancer cells. Disrupting homeostasis of mitochondria and ER are followed by chrysin in apoptosis induction in cancer cells. Autophagy is another programmed cell death that is activated by chrysin in cancer therapy. As autophagy has a dual role in cancer, meaning it may suppress cancer progression, or may function as a pro-survival factor in promoting the proliferation of cancer cells [330,331,332,333], much attention should be directed towards the regulation of autophagy by chrysin in cancer therapy. It has been reported that chrysin induces autophagy in cancer therapy, showing the anti-tumor role of autophagy. However, more studies will reveal a relationship between chrysin and autophagy in cancer therapy. In terms of molecular pathways, oncogenic ones, such as STAT3, NF-κB, and PI3K, that are involved in cancer growth and metastasis, are suppressed upon chrysin administration. MiRs are also potential targets of chrysin in cancer therapy that their expression is regulated. Noteworthy, since studies have shown that chrysin is capable of modulating the expression of miRs, further studies can focus on evaluating the effect of chrysin on other types of non-coding RNAs, such as long non-coding RNAs (lncRNAs) and circular RNAs (circRNAs).
Another potential application of chrysin is in suppressing chemoresistance. One of the major challenges in the field of chemotherapy is the resistance of cancer cells into the inhibitory effect of currently applied chemotherapeutic agents. Chrysin induces apoptosis to sensitize cancer cells into chemotherapy. Moreover, molecular pathways, such as Nrf2, that induce chemoresistance, are suppressed via chrysin. Further studies can focus on revealing other molecular pathways, such as miRs in chemoresistance, and the role of chrysin in their regulation.
In fact, different aspects of cancer cells are affected by chrysin, including proliferation, metastasis, and chemoresistance. These inhibitory effects are mediated via affecting both molecular pathways and mechanisms that were comprehensively discussed in the main text. As poor bioavailability is one of the drawbacks of chrysin in cancer therapy, a section was allotted to examine the role of nanoparticles for promoting bioavailability and the therapeutic effects of chrysin in cancer therapy. It is worth mentioning that these results were based on in vitro and in vivo experiments. Further studies can focus on evaluating the role of chrysin in clinical studies, which is important for clinical translation of chrysin.
This entry is adapted from the peer-reviewed paper 10.3390/biom10101374
