1. Native Italian Trouts and the Taxonomy of the “Peninsular Trout”
The short description and illustration of
S. cenerinus [
29] may correspond to the pelagic morph of several anadromous
Salmo species ([
33,
34], pers. obs.). Nardo [
35] modified his previous view [
29], raising doubts on the original description of
S. cenerinus, accepting the view of [
36] (cited as 1858 by [
35]), and eventually considering this taxon as a synonym of
Trutta fario L. (=
S. trutta). Heckel and Kner [
36] reported only two trout species from the Venetian Provinces:
Salar Ausonii Valenciennes 1848 [
37] (=
Trutta fario L. sensu [
35]) and
Fario carpio (=
Trutta carpio sensu [
35] =
S. carpio) from the Garda Lake. While
Salar genivittatus Hecker and Kner 1858 was subsequently recognized as a morph of
S. marmoratus [
38], Heckel and Kner [
36] considered
S. marmoratus as a color morph of
Salar Ausonii. Therefore, Nardo [
35] likely considered the marble trout of this area as color morphs of
Trutta fario.
Kottelat [
38] assigned
S. cettii to the native peninsular Tyrrhenian and southern Italian trout, including islands, and “tentatively” assigned
S. cenerinus to the native north-Italian (Adriatic) peninsular trout. Consistently, he did not consider
S. cenerinus as jun. syn. of
S. marmoratus, since “there would be no available name for the present species and it should be either listed as
Salmo sp. or a new name should be created for it”. Kottelat [
38] also synonymized
S. ghigii with
S. cettii apparently only because Pomini [
31] was unable to discriminate the trouts of the Sagittario River from the Sardinian trouts. Kottelat and Freyhof [
39] accepted the point of view of Kottelat [
38], while noting that “recent studies (…) suggest that the trouts of Sicily (…) belong to a distinct molecular lineage (…). If confirmed, this lineage should retain the name
S. cettii; the name
S. ghigii would probably be the valid name of the others”.
Using combined mitochondrial (mtDNA) and nuclear (nDNA) markers, Segherloo et al. [
12] found a close relationship between the Sicilian trout of Val di Noto and Atlantic
S. trutta. A consistent result was found by another nuclear phylogenetic study of the Moroccan trouts, which included the Sicilian trout of Val di Noto in a robust “Afro-Atlantic clade”, likely originated from a colonization wave of an Atlantic lineage from Iberia (“Duero” lineage; [
40]). The only North-African sample analyzed by Segherloo et al. [
12], that these authors tentatively assigned to
Salmo pellegrini Werner 1931, is closely related to Mediterranean and Adriatic brown trouts, thus clearly belonging to a different lineage; this sample comes from the Oum er-Rbia River, where Snoj et al. [
40] identified trouts of an “Atlas clade”. Several studies showed that the Sicilian trout is morphologically distinct from other Italian trouts [
30,
41,
42,
43,
44]. Mitochondrial phylogenies also show that the Sicilian trout is included in a clade of North-African trouts, which also includes the sequenced types of
S. macrostigma [
45] and the Atlantic trout lineage, called the “Southern Atlantic clade” [
22,
46]. However, no nuclear or combined mitochondrial and nuclear phylogenies were ever constructed including the types of
S. macrostigma, which may be unrelated to the Afro-Atlantic clade.
Rafinesque-Schmaltz [
26] described
S. cettii from two trout populations: Val di Noto and Val Demone. The molecular phylogeny of
S. cettii has been investigated only using the former population, since no genetic samples have ever been collected and analyzed from the Val Demone, which has likely been extirpated [
47]. The recovery and analysis of any such molecular sample (e.g., from a museum lot) would have important consequences on the scientific names of Italian trout lineages. There are three possible scenarios: (i) the Val Demone population belongs to an undescribed endemic trout lineage; (ii) the Val Demone population is conspecific with the peninsular trout; (iii) the Val Demone and Val di Noto populations are conspecific.
With the limitation of substantial sample biases, several studies did not find genetic or ecological discontinuities between native northern (South-western Alps [
49]) and central-southern peninsular trout lineages that would justify the designation of different taxa, except
S. carpio and
S. fibreni [
20,
32,
50,
51,
52,
53,
54]. Segherloo et al. [
12] assigned trout samples of the upper reaches of the Po drainage to
S. cf.
cenerinus, and samples of the Zrmanja and Mornos basins (Balkan peninsula) to
Salmo farioides Karaman 1938, in the same area of its type locality (Krka River, Croatia [
55]; neotype designated by Bianco [
56]).
S. cenerinus was found in brackish conditions [
28,
29], however the only native Italian trout recorded in the sea is
S. marmoratus [
57]. Further, there presently are no known native populations (nor genetic traces of past populations) of peninsular trout in the area where
S. cenerinus was described. On the other hand, anadromous non-native populations of
S. trutta, including hybrids, are known to occur in the Adriatic region, including Italian waters [
58,
59]. Chiereghin died in 1820, and likely described
S. cenerinus from the late 1700s to the early 1800s. Fish culture projects, possibly including non-native
S. trutta, started in this area in the second half of the 19th century [
35]. On the other hand, the hypothesis that the trout described by Chiereghin was a pelagic morph of
S. trutta cannot be ruled out, since introductions of non-native salmonids, possibly including
S. trutta, have occurred in northern Italy at earlier times (
Section 3). In the 1970s, Borroni and Grimaldi [
8] just reported that introductions of non-native
S. trutta had been occurring “for decades” in Italy. Bianco and Delmastro [
60] and Bianco [
56] synonymized
S. marmoratus and
S. cenerinus based on the illustration of
S. cenerinus [
28], its anadromous habits [
28,
29], and information gleaned from Gridelli [
61], who reported only the presence of the marble trout in the Venezia Giulia Region, previous to stocking activities of non-native brown trouts, which started in 1934. However, Nardo’s [
29] Venetian Provinces of the 1850s (type locality of
S. cenerinus) are geographically distinct from Gridelli’s [
61] Venezia Giulia Region of the 1930s [
62]. Bianco [
56] synonymized
S. ghigii with
S. farioides, however: (i) no neotype of
S. ghigii was designated, likely due to the difficulty of finding “purebred” individuals in the type locality; (ii) no molecular analyses were conducted; (iii) the synonymy was essentially based on coloration patterns and biogeographical reconstructions. Therefore, we choose not to consider
S. ghigii as a junior synonym of
S. farioides. As a result, until evidence is provided of introductions of non-native
S. trutta in the area where
S. cenerinus was described, we consider
S. cenerinus as jun. syn. of
S. marmoratus sensu Bianco [
56]. While genetic differences between peninsular-trout populations have been found at different geographic scales [
23,
63], until more comprehensive genetic and ecological data are made available on Tyrrhenian and Adriatic native Italian trouts, we tentatively consider
S. ghigii as a valid name for all the populations of Italian peninsular trout, sensu Zanetti et al. [
64] and Lorenzoni et al. [
65].
2. Phylogeny and Phylogeography of S. marmoratus
Nuclear phylogenetic reconstructions and molecular clocks defined a robust
S. marmoratus clade, including two distinct northern and southern Adriatic clades that diverged ~0.84 ± 0.4 million years ago (mya) [
66,
67], and whose taxonomic status has not yet been evaluated. Pustovrh et al. [
67] showed that
S. marmoratus is closely related to a nuclear “
S. trutta complex” lineage, including several clades associated with different brown-trout taxa, and estimated the divergence between these two lineages at 1.4 ± 0.8 mya (2.2–0.6 mya). A fossil-calibrated nDNA phylogeny estimated an earlier divergence, at ~4–5 mya [
68]. An extensive molecular phylogeny combining nDNA and mtDNA sequences rooted with
S. salar, essentially consistent with previous nDNA phylogenetic reconstructions, supported
S. marmoratus as a phylogenetic species of possibly hybrid origins, sister to a clade including >20
Salmo species [
12].
In northern Italy, northern Adriatic
S. marmoratus populations are strongly associated with the “Marmoratus” (MA) haplogroup of the mtDNA control region (D-loop) [
22,
69,
70,
71,
72]. However, MA haplotypes have also been found in several brown trout taxa and populations of Greece, Albania, Croatia, central Italy, and Corsica, e.g., [
23,
48,
66,
71]. Like several other brown trout taxa and populations, in the Balkans Southern Adriatic
S. marmoratus populations can be associated with the “Adriatic” (AD) mtDNA haplogroup [
67,
72].
Mitochondrial molecular clocks estimated much more recent origins of the MA and AD haplogroups (0.21–0.05 mya and 0.39–0.13 mya, respectively, considering the 95% highest probability density intervals estimated using two different substitution rates [
23]) than the time of divergence between
S. marmoratus and the nuclear “
S. trutta complex”. It was suggested that the observed mitochondrial-nuclear phylogenetic discordance might be the effect of incomplete lineage sorting or asymmetric introgressive hybridization (mtDNA capture; e.g., [
73]). The much older time of divergence between these lineages relative to the time of haplogroup differentiation strongly supports the latter mechanism. Paleointrogressive hybridization between the marble trout and the Apennine Mediterranean trout could have occurred during several secondary contacts as a consequence of the expansion of the Po paleo-basin during glacial maxima, as it occurred in other
Salmo species [
22,
32,
53,
59,
66,
74]. Mosaic distributions of mtDNA haplogroups among different taxa are common also in areas without a history of non-native trout’s stocking (e.g., Albania [
75]), and similar distributional and diversity patterns might have occurred in Italy after the Last Glacial Maximum (LGM ~18,000 years ago).
Phylogenetic patterns, molecular clocks and the zoogeography of congeners suggest that
S. marmoratus is one of the
Salmo lineages that diverged in the paleo-Adriatic drainage, in freshwater refuges formed during the preceding Lago Mare phase (~5 mya). During the Pleistocene, reduced salinity, cooler sea temperatures, and extensive palaeo-river basins would have facilitated the westward dispersal of these freshwater lineages across the region through multiple waves of colonization, bottlenecks, and secondary contacts [
32,
72,
75], allowing
S. marmoratus to colonize the orographic left tributaries of the palaeo-Po basin [
56,
76,
77]. After the LGM, increased salinity levels and sea-level rise disconnected these populations, facilitating allopatric fragmentation and differentiation of mtDNA lineages, resulting in the present geographic distribution [
32].
In the northern Adriatic basin,
S. marmoratus exhibit a west-to-east geographic gradient in MA-s1 and MA-s2 haplotype distribution, consistent with the described stepwise westward migration and phylogeographic scenario [
78]. Significant microgeographic genetic differentiation was also measured within basins, e.g., between rivers and their tributaries, suggesting the presence of limited gene flow among different populations [
79,
80]. A contact zone between
S. marmoratus and
S. ghigii was found in the South-western Alps (
Section 3).
3. Presence of S. ghigii in the Italian Alpine and Subalpine Region
Within the Italian Alpine region [
81], viable native populations of
S. ghigii have only been found in the South-western Alps (Cottian and Maritime Alps: upper Stura di Lanzo, upper Dora Riparia, upper Chisone, upper Pellice, upper Po, upper Stura Demonte, upper Gesso, and upper Tanaro basins), where a contact zone with
S. marmoratus was described, along a geographic distribution gradient of genetic variants associated with different trout phyletic lineages [
20,
21,
22,
23,
34,
48,
50,
77,
80,
82,
83];
Section 2. The MA, AD, and “Mediterranean” (ME) haplotype probability densities relative to elevation show an altitudinal zonation suggesting local habitat differentiation between the two parapatric species, with
S. marmoratus being dominant at 0–1000 m above sea level (asl) and
S. ghigii at 1000–2000 m asl [
23]. These findings are consistent with the distributional patterns of trouts with different phenotype, described in some historical accounts [
84,
85,
86].
The South-western Alps are a known glacial refuge, where native populations of
S. marmoratus and
S. ghigii could have survived the LGM [
82]. Introgression rates of alien Atlantic genes into native trout populations are here highly variable (0–70%; [
20]). In contrast, in most of the North-western and South-eastern Alps [
81] only the lower tracts of the rivers were unaffected by the ice cap during the LGM. Assuming that
S. ghigii and
S. marmoratus exhibited a habitat segregation pattern analogous to that presently observed in the South-western Alps, the LGM likely allowed the survival of
S. marmoratus at lower altitudes, while
S. ghigii might have been pushed into the marble trout habitat and outcompeted [
82]. After the last glaciation, most Alpine lakes and headwaters may have only marginally been colonized by
S. marmoratus and likely remained “fishless” (i.e., troutless). In historical times, these systems were artificially stocked with translocated salmonids, including non-native Atlantic
S. trutta [
82], to support subsistence and recreational fisheries [
87,
88,
89]. The capacity of
S. marmoratus to outcompete other trout species was observed by Sommani [
90], who observed that in specific water courses marble trout is able to rapidly replace brown trout (
S. trutta fario =
S. ghigii or
S. trutta; this author was unable to discriminate between these species), when restocking practices are interrupted.
In the Lake of Garda basin, a known glacial refugium [
91] in the South-eastern Alps [
81], a study [
92] found traces of the mitochondrial variant ADcs-1 (the most widespread AD haplotype [
23], typically associated with the “Adriatic grouping” of
S. trutta fario, sensu [
50] =
S. ghigii). The prehistoric presence of
S. ghigii in the Lake Garda refugium is also consistent with the presence in
S. carpio of haplotypes phyletically related to haplogroups typically associated with
S. ghigii (AD) and
S. marmoratus (MA), suggesting that one or more paleohybridization events occurred in this basin between these trout lineages [
20,
23,
50,
53]. This also supports the hypothesis of extensive secondary contacts and hybridization events between peninsular and marble trout lineages before the last glaciation in this region [
53]. The ADcs-1 haplotype was also found in two museum specimens with lacustrine morphs collected in Lake Garda and Lake Maggiore in 1877 and 1879, respectively [
83]. Lake Maggiore is another known glacial refugium [
91,
93] located in the North-western Alps [
81]. The presence of AD haplotypes in these basins suggests that relict populations of
S. ghigii might have survived the LGM in other glacial refugia of the North-western and South-eastern Alps. More speculatively, since
S. marmoratus is the only native trout with lacustrine morphs in this region, this might also indicate the more common presence of marble trouts with AD haplotypes in this basin in past historical times, or even the presence of recently extinct and undescribed trout taxa [
20,
94,
95].
With the only exception of the South-western Alps, the absence of viable populations of
S. ghigii in northern Italy clearly indicates that all relict populations of
S. ghigii that might have survived to the LGM in other glacial refugia were subsequently extirpated. This might have reasonably occurred due to demographic or genetic swamping [
96] caused by the man-made massive and prolonged introductions of non-native
S. trutta in historical times. Consistently, introgression rates of Atlantic
S. trutta into
S. marmoratus are higher in the North-western and South-eastern Alps, and only traces of the haplogroups typically associated with native
S. ghigii were found [
21,
82]. This scenario is supported by the probabilistic approach adopted by [
92], which showed that, in spite of the massive introductions of
S. trutta, genetic traces of extirpated
S. ghigii populations could still be found in some glacial refugial areas such as the Lake Garda basin. Such dramatic effects could have been facilitated by strong numerical differences between native
S. ghigii populations and
S. trutta introductions, low hybrid fitness, and weak reproductive barriers. By contrast, the presence of partial reproductive barriers between non-native
S. trutta and
S. marmoratus [
80,
97], the competitive advantage of
S. marmoratus [
90], and marble-trout stocking could have prevented the lineage or local genomic extinction of the latter species. In spite of the presence of high introgression rates [
82], neither demographic swamping nor local genomic extinctions of native Apennine
S. ghigii have ever been described in the Tuscano-Latium Italian ichthyogeographic region, where non-native
S. trutta have been and are being introduced. On the other hand, these
S. ghigii populations were much less impacted by habitat modification or competition with other species during the LGM, and were likely larger and less fragmented when they were flooded by
S. trutta introductions.
Some studies found the allozymic variants LDH-C1*100 and TF*102, typically associated to
S. ghigii populations in trout populations native to France and south-west Piedmont, in sites collected east of the South-western Alps, hence suggesting the presence of
S. ghigii outside the mentioned contact zone with
S. marmoratus [
23]. However, these allozymes were also found at high frequency in Danubian native populations of different
Salmo species [
49,
98,
99]. In one of these studies, Largiadèr and Scholl [
100] assumed the native status of an “Adriatic fario” in a large portion of the Po basin, based on molecular studies conducted in south-western Piedmont [
49] and on phenotypic studies that were however unable to discriminate between Atlantic (
S. trutta) and Adriatic (
S. ghigii) trout phenotypes [
101]. These authors found these two allozymic variants at high frequency (~20–30%) in Engadin (Danubian basin), in the Müstair, tributary of the Adige River, and in the Poschiavo valley (Po basin, Poschiavino Torrent, tributary of the Adda River); and at low frequency (~0–10%) in the Ticino and Valais basins, including a tributary of the Diveria Torrent (Chrummbach). No “purebred” individuals were found. In fact, all these populations had been directly or indirectly either entirely replaced or heavily stocked with trout lineages of the Danubian basin via the Poschiavo hatcheries, for at least one century before the study collection [
100]. This would explain the genetic similarities between the trouts of the Poschiavino and Ticino valleys, subsequently detected by other studies using microsatellite and AFLP markers to investigate the adaptive divergence and phylogeographic patterns of trout populations of the Rhine, Rhone, and Po basins [
102,
103]. Just like [
100], also these studies assumed the presence of an “Adriatic trout” (
S. cenerinus, sensu [
39] =
S. ghigii;
Section 1) in the Poschiavino and Ticino valleys, based on the literature [
39,
100,
101]. However, given the lack of genetic references (allele size range) of Danubian trout populations (possibly
Salmo labrax Pallas 1814 [
102,
103]) it is not possible to know whether the observed “Adriatic” genetic traces in the Poschiavino and Ticino valleys were originally present in this region, or were left by introduced Danubian stocks [
102], as also suggested by the presence of Danubian mtDNA haplotypes (DA haplogroup) in the Ticino basin [
78]. Keller et al. [
102,
103] also found evidence of introgression of the Poschiavo population into one Rhine population (SE). SE is the closest Rhine population to the Danubian drainage, suggesting the presence of stocking activities and translocations between SE, Poschiavo, and Danubian systems.
There are several descriptive accounts (cuisine recipes, anecdotes, poetry, and even paintings e.g., [
104]) of trouts in the North-western and South-eastern Alps (e.g., Lakes of Como and Garda basins) before the dramatic expansion of the fish-culture industry that promoted the rapid diffusion of the non-native Atlantic
S. trutta in the early 19th century (1850–1893, [
10]). Several ones [
105,
106,
107,
108,
109,
110,
111,
112] depict or describe trouts without a marbled coloration pattern and with either red and black dots, phenotypically compatible with several trout taxa, or with speckled dark patterns on a silvery background, compatible with a generalized pelagic (lacustrine) morph of anadromous trout. Adult
S. marmoratus living in rivers typically exhibit a marbled coloration pattern [
113]; however, anadromous individuals in pelagic conditions can exhibit a silvery and dark-speckled coloration pattern, even leading to taxonomic confusion, e.g., [
114].
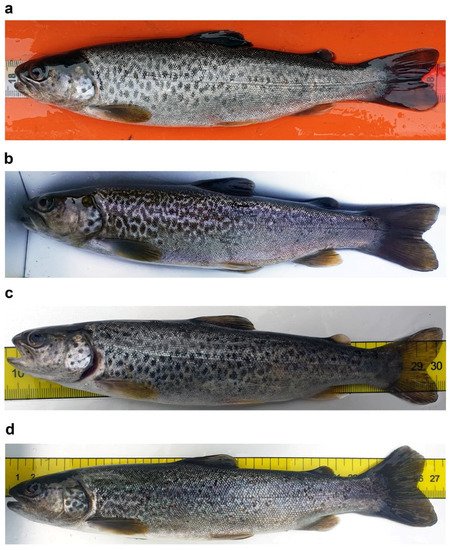
Young marble trout typically exhibit an irregular black or red-and-black dotted pattern, with a large black preopercular blotch, similar to adult brown trouts [
113] (
Figure 2a); the dark dotted pattern can change to a marbled pattern in a few months in subadults (
Figure 3a–d); and adults living in small and fast-flow streams can become reproductive at half the typical length at maturity, while retaining a “brown-trout” red or red-and-black dotted pattern [
115] (
Figure 2b,c).
Figure 2. Examples of dotted coloration patterns in
S. marmoratus: juvenile coloration pattern (
a), and adults living in small and fast-flowing streams (
b,
c); (
a) juvenile from Roledo (Piedmont, Verbano-Cusio-Ossola: VCO; 46°10′16.7″ N 8°18′49.7″ E), 15.5 cm total length—TL, 29.0 g wet mass, 22 months of age, black-and-red dotted pattern, MA haplogroup, q
Ma 0.995 (admixture proportion of a cluster including purebred
S. marmoratus references), 90% BCI 0.966–1.000; (
b) adult (reproductive) specimen from Rio Ischielle, tributary of the Avisio Torrent (Province of Trento); the specimen was collected from a population which resided for 2 generations in this small stream, which descended from hatchery-reared
S. marmoratus with marbled phenotype collected from the Adige River [
115]; 26.9 cm TL, courtesy of Leonardo Pontalti; (
c) adult (reproductive) specimen from Rio della Balma, tributary of the Sangone River (Province of Torino), 18.5 cm TL, MA haplogroup, q
Ma 0.996, 90% BCI 0.978–1.000, courtesy of Paolo Lo Conte.
Figure 3. Examples of dotted coloration patterns in S. marmoratus: conspicuous ontogenetic chromatic variation in pit-tagged individuals which were recaptured at different times; (a,b) subadult specimen sampled in Roledo (Piedmont, Verbano-Cusio-Ossola: VCO; 46°10′16.7″ N 8°18′49.7″ E), age and genetic data unavailable; (a) sampled on 28 April 2021, 20.0 cm TL, 86 g, dotted pattern; (b) recaptured in the same site on 28 October 2021, 23.4 cm TL, 122 g, marbled pattern; (c,d) Subadult specimen sampled in Prata di Vogogna (Piedmont, VCO; 46°1′40.8″ N 8°17′2.2″ E), age and genetic data unavailable; (c) sampled on 26 April 2021, 20.6 cm TL, weight not available, dotted pattern; (d) recaptured in the same site on 19 October 2021, 26.4 cm TL, 166.0 g, marbled pattern.
On the other hand, there is ample evidence of salmonid introductions in old historical times from outside Italy. Domestication practices and translocations of freshwater fishes, even across mountain ranges, go back to the Middle Ages and possibly to the Neolithic, seamlessly continuing through to the 18th and 19th century, before the onset of the fish-culture industry [
10,
116,
117,
118,
119]. Non-native trouts with “brown-trout” dotted coloration patterns could have been introduced in northern Italy from adjacent areas such as the orographic right tributaries of the Po River, or even beyond the Alpine Divide, e.g., from the Danube basin, such as the common carp
Cyprinus carpio L. in the Roman Period [
10,
120].
This entry is adapted from the peer-reviewed paper 10.3390/biology11040576