1. Calcium Mediated Exocytosis
Astrocytes are essential in modulating neuronal activity and synaptic neurotransmission [
20]. Nerve terminals are encased with astrocytes and are strategically located to communicate effectively with synapses [
20]. The astrocytic responses towards synaptic stimulation have been well established [
21]. They mediate Ca
2+ dependent glutamate release and regulate synaptic neurotransmission [
22]. As the intracellular Ca
2+ level required for astrocytic glutamate release is within physiological limits, this release can be exploited as a signaling mechanism to alter synaptic neurotransmission and plasticity within the CNS [
23]. Primarily, the generation of intercellular Ca
2+ waves (ICW) involves the release of Ca
2+ from the ER via G protein coupled receptor (GPCR) activation [
24]. Studies on glia revealed the presence of mGluRs which, upon activation by physiological ligands, resulted in the synthesis of inositol 1,4,5-triphosphate (IP3) and subsequent Ca
2+ release [
25]. This release of Ca
2+ promoted the onset and maintenance of ICW of the glial cell, which provided long-range signaling [
26]. These waves depict the rise in Ca
2+ levels in the cytoplasm, which communicates with other cells and has a wave-like appearance that radiates from its originating source. The ICW is initiated by the release of ATP that follows after hemichannel opening [
27]. Interestingly, it was demonstrated that glutamate concentration was directly proportional to the frequency oscillations of Ca
2+ waves [
28]. This Ca
2+ is released by the hippocampal astrocytic cells from intracellular storage both naturally and in response to the activation of Gq-linked GPCR by binding IP3 to its receptor (IP3R). The released Ca
2+ in astrocytes is essential and sufficient for the secretion of gliotransmitters, such as ATP and glutamate, and further affects the neuronal activity. IP3R type 2 (IP3R2) appears to be the major IP3R expressed by astrocytes [
29]. IP3R-mediated Ca
2+ signaling is speculated to cause the activity-dependent and selective release of chemical transmitters. In astrocytes, IP3R2 was once the only known Ca
2+ channel; however, Ca
2+ imaging techniques have recently determined new Ca
2+ sources including mitochondria [
30]. Rakers et al. reported that the release of IP3R2-dependent Ca
2+ from internal reserves causes a rise in astroglial Ca
2+ during neurological disorders including stroke [
31]. Astrocytes emit several signaling chemicals such as glutamate, D-serine and ATP. Activities of these molecules, such as modulating synaptic transmission and influencing particular behavior, are vigorously studied, but the identity of their cellular compartments remains unknown [
32]. The pharmacological inhibition of vesicular glutamate transporters (VGLUTs) significantly decreased exocytotic glutamate release from astrocytes which is a Ca
2+ dependent phenomenon, indicating that these transporters may be instrumental in the astrocytic glutamate release in CNS. VGLUTs transfer cytoplasmic glutamate into exocytotic vesicles, which are propelled by a proton gradient created by vacuolar ATPases (V-ATPases) [
33]. VGLUT1 and 2 are also seen, along with synaptic-like vesicles [
34]. Montana et al. demonstrated that VGLUTs 1 and 2 are present in rat astrocytes and show high immunoreactivity, justifying their role in the glutamate release via exocytosis [
35]. Conversely, the VGLUT mRNAs were absent in astrocytic transcriptome [
36]. This supported the notion made by Li et al. that VGLUTs were absent in the astrocytes [
37]. The cytosol of astrocytes have high glutamate concentrations ranging from 0.1–5 mM, but extracellular glutamate levels lie within the sub-micromolar range [
38]. In astrocytes, glutamate is packed into synaptic-like vesicles and is released in a Ca
2+ dependent mechanism, demonstrating the contribution of astrocytes in glutamatergic transmission.
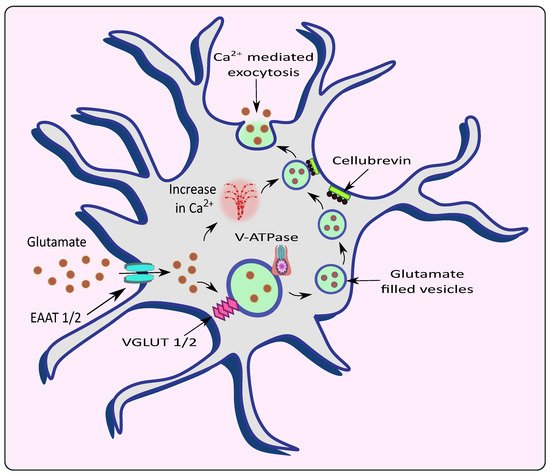
Astrocytes exert controlled glutamate exocytosis via a protein complex called the soluble N-ethylmaleimide-sensitive factor (NSF) attachment protein (SNAP) receptor (SNARE) complex, which regulates vesicle fusion [
39]. Synaptobrevin 2, Syntaxin 1, and synaptosome-associated protein of 23 kDa are all part of the core SNARE complex (SNAP-23), while Synaptotagmin 4 is a Ca
2+ sensor. SNARE proteins are found on both the vesicular membranes and the presynaptic plasma membranes that cause membrane fusion [
40]. In the neurons, the vesicle-associated membrane protein 2 (VAMP2) binds to Syntaxin and synaptosomal-associated protein 25 (SNAP25) on the cell membrane to form the SNARE complex. Synaptotagmin 1, a Ca
2+ sensor expressed by neurons, detects the Ca
2+ rise caused by Ca
2+ entry via voltage-gated Ca
2+ channels and triggers the fusion of vesicles to the cell membrane, releasing glutamate. VAMP2/VAMP3, Syntaxin and SNAP25/SNAP23 are all expressed by astrocytes with similar functions [
41]. However, Bezzi et al. suggested that, instead of VAMPs, astrocytes express cellubrevin—a SNARE complex of astrocytic vesicles [
34]. Further, research revealed that astrocytes produce Synaptotagmins 4, 7, and 11, which cause the release of glutamate from vesicles in response to a rise in intracellular Ca
2+ levels in similar fashion to neurons. Intracellular Ca
2+ levels need to rise in the range of 250 to 350nM to stimulate astrocytic glutamate release [
41,
42,
43]. With an increase in Ca
2+ levels, the vesicles fuse with SNARE proteins and undergo Ca
2+-mediated exocytosis to release glutamate [
44,
45] as shown in
Figure 2. Increasing Ca
2+ concentrations by overstimulation of glutamate receptors could lead to excitotoxicity and neuronal death [
46]. Therefore, impairment in Ca
2+ signaling could lead to the progression or worsening of neurodegenerative diseases such as AD and PD.
Figure 2. Release of glutamate from astrocytes via Ca2+ mediated exocytosis. Astrocytic EAAT promotes the uptake of glutamate from the synaptic cleft which is filled into the vesicles by VGLUT in the presence of V-ATPase. The rise in intracellular Ca2+ causes the vesicles to fuse with the membrane with the help of an astrocytic vesicular SNARE protein cellubrevin and promotes Ca2+ mediated exocytosis of glutamate.
AD is associated with progressive neurodegeneration and marks its presence primarily through cognitive deficits in patients. Its hallmarks, namely neurofibrillary tangles (NFTs) and the occurrence of amyloid-beta (Aβ) plaques, have been blamed for the progression and worsening of AD [
47]. Mostly, it is believed that AD pathology occurs through the amyloid cascade hypothesis originating via the amyloid precursor protein (APP). The action of enzymes namely β secretases and γ secretases produce insoluble Aβ that confers neurotoxicity [
48]. Epigenetic modification, proteolysis abnormalities, oxidative stress, neuroinflammation, hampered mitochondrial function, and faulty autophagy are some of the variables that contribute to accelerated aging and neurodegenerative disorders [
49,
50,
51,
52]. However, in recent years, the pathology of AD has expanded in multiple dimensions including the pathogenic role of dysfunctional glial cells and the excessive release of neurotransmitters such as glutamate [
53].
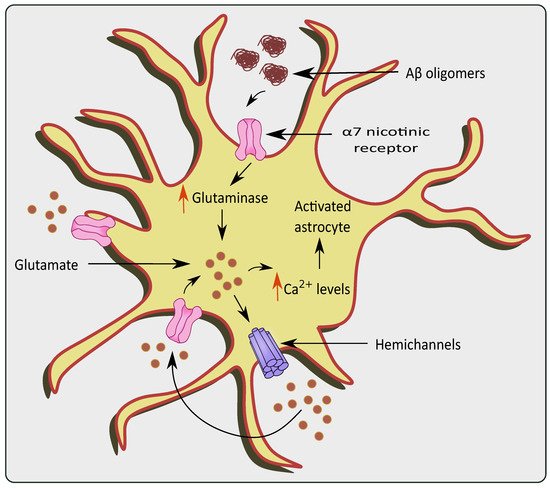
Neurotoxicity occurs due to an over-accumulation of extracellular glutamate during Aβ aggregation [
54,
55]. The effect of Aβ 1–42 on a α7 subunit containing nicotinic receptors (7nAChR) could also increase internal Ca
2+ currents and subsequent glutamate uptake/release causing glutamate excitotoxicity as shown in
Figure 3 [
56]. Similarly, Aβ 1–42 has picomolar affinity for the 7nAChR, which is known to enhance glutamate release when activated [
57,
58]. Lower levels of endogenous Aβ 1–42 are necessary for normal brain function, while at a higher concentration the resultant accumulation and aggregation results in neurotoxicity [
59] Through the (7nAChR), Aβ 1–42 can cause glutamate release in the hippocampal nucleus, which is cleared from the extracellular space quickly (msec) by high-affinity EAATs [
60,
61]. Nicotine-induced glutamate release via the 7nAChR is supported by the hypothesis that Aβ 1–42 binding near the nicotinic site on the 7nAChR can elicit glutamate release [
58]. Furthermore, increased Aβ 1–42 synthesis proportionately increases glutamate release in the Cornu Ammonis (CA) 1 area of the amyloid precursor protein/Presenilin 1 (APP/PS1) mouse model [
62]. Lower density of 7nAChRs in the CA3 region could explain why an elevated Aβ 1–42 concentration is required to elicit greater glutamate release in the hippocampal region. Aβ 1–42 protein deposition has been reported to occur initially in the CA1 and DG, followed by the CA3 in patients with AD [
63]. Hascup and colleagues discovered that enhanced Aβ 1–42 evoked glutamate release in the CA1 and DG at lower doses [
64]. The presence of Aβ causes the activation of 7nAChRs present in the astrocytes of the hippocampal regions [
65]. Similarly, 7nAChR over expression was observed in the rat astrocytes in the presence of AD pathogenesis [
66]. It is evident that 7nAChRs elevate the intracellular Ca
2+ levels by stimulating Ca
2+ release from intracellular reserves of astrocytes [
67].
Figure 3. Glutamate excitotoxicity via overstimulation of α 7 nicotinic receptors in the presence of AD pathologies. The presence of Aβ oligomers causes activation and over-stimulation of α 7nAChRs, which increase levels of glutaminase and glutamine in the astrocytes. This causes a rise in Ca2+ levels, ultimately stimulating hemichannels to release more glutamate causing glutamate excitotoxicity.
Similarly, the actions of Aβ 25–35 on the astrocytic purinergic receptors promote Ca
2+ level elevation [
68]. Chronic calciumopathy, observed in AD, affects neuronal Ca
2+ homeostasis and Ca
2+ signaling [
69]. In AD, the senile plaques promote Ca
2+ hyperactivity in astrocytes that trigger excessive glutamate release. The released Ca
2+ from the endoplasmic reticulum is essential for astrogliotic response. Therefore this higher Ca
2+ signaling, as seen in the entorhinal region and prefrontal cortices of rodent AD models, suggests the abnormalities associated with these pathologies [
70]. Additionally, enhanced astroglial Ca
2+ signaling followed by glutamate excitotoxicity has been seen in mice with the APP/PS1 gene [
71,
72]. Recently, Pham et al. found that astrocytic Aβ exposure resulted in both Ca
2+ dependent and independent glutamate release, which caused excitotoxicity. Interestingly, a notable amount of glutamate was released before the Ca
2+ elevation during Aβ administration, followed by a surge in glutamate with a subsequent rise in Ca
2+ [
68].
PD is another neurodegenerative disease with impairment in neurons present in the dopaminergic system [
73]. The region of substantia nigra pars compacta (SN) residing within the midbrain is highly affected. Pathological hallmarks such as Lewy bodies and α synuclein deposition are considered to play a role in the progression of PD [
74]. Patients diagnosed with PD mainly show tremors, muscle rigidity, motor deficits, gait instability, and memory deficits [
75]. Recently, autonomic dysfunction has been attributed to PD pathology [
76]. Impaired homeostasis of neurotransmitters such as glutamate also plays a pivotal role in the pathogenesis of PD [
77]. Inflammatory processes-induced astrocytic glutamate excitotoxicity has also been linked to PD, which causes changes in glutamate transporters and receptor expression [
78,
79]. Moreover, the accumulation of α-synuclein increases the Ca
2+ depolarization-dependent release of presynaptic glutamate as demonstrated using synaptoneurosomes obtained from the forebrain [
80]. The α-synuclein releases glutamate in a Ca
2+ dependent mechanism, which further activates the extrasynaptic NMDA receptors, and causes neuronal damage [
81]. This increase in glutamate activated the AMPA receptors that further upregulate the glutamate release [
82]. Interestingly, α-synuclein mobilizes the glutamate vesicles from the pool of reserves [
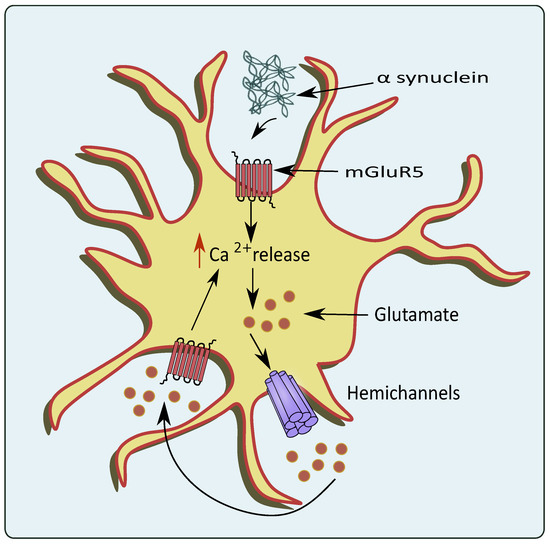
83]. mGluR5 overexcitation upon the binding of α-synuclein also stimulates the release of Ca
2+ leading to glutamate excitotoxicity as shown in
Figure 4 [
84]. A small protein, DJ-1 encoded by
PARK7 gene has also been associated with PD pathogenesis, and knockout of
PARK7 hampered glutamate uptake via astrocytes. This altered glutamate uptake was associated with the downregulation of EAATs, which led to neurotoxicity in PD patients [
85]. Similarly, Wang et al. showed that JWA knockout mice in pathologies of PD reduced the glutamate uptake by hampering EAAT 2 expression [
86].
Figure 4. Glutamate excitotoxicity mediated through overexpression of mGluR5 by PD pathologies. In the conditions of PD, the α-synuclein activates astrocytic mGluR5 that elevates intracellular Ca2+ levels and stimulates the release of glutamate through the hemichannels.
ALS is a neurodegenerative disease that involves the degeneration of motor neurons in the CNS [
87]. Its prominent characteristic features include motor weakness and loss of motor neurons, gliosis and atrophy of skeletal muscles [
88]. Significantly reduced expression of GLT-1 in the motor cortex and spinal cord has been proposed to be one of the main factors leading to glutamate excitotoxicity in ALS [
19].
Metadata analysis of studies of transgenic ALS cell cultures suggests that extrinsically boosting the astrocytic GLT-1 level before ALS end-stage may improve the reuptake of glutamate and could be considered a therapeutic strategy. Excitotoxicity could be perhaps due to the ability of those ALS astrocytes that render themselves susceptible to even minor alterations in the neuronal environment. Further, at pre-onset, lowering astrocytic GluR1 levels could help to lessen intracellular Ca
2+ [
89]. Mutations in valocin containing protein (VCP) genes are causative for ALS. VCP mutant astrocytes showed reduced glutamate uptake and induced reactive astrocytes. This could serve as a protective mechanism at first but becomes toxic over time due to impaired homeostasis. These ALS astrocytes up-regulate inflammatory signaling and are seen to reduce its supportive actions to neurons [
90].
In a mouse model of ALS, an increased influx of Ca
2+ has been shown to be instrumental in glutamate exocytosis by affecting vesicle fusion and release mechanisms [
91]. Furthermore, SOD1 gene mutations in familial ALS interfere with mitochondrial function and prevent glutamate reuptake, thus causing glutamate excitotoxicity [
27]. Another interesting target seen in familial ALS is the upregulation of the ATP- binding cassette transporters (ABC) transporter glycoprotein (P-gp). It is known to be upregulated by NMDA receptors activated by glutamate that is excessively secreted by astrocytes with mutant superoxide dismutase 1 (SOD1) [
92]. The sporadic and familial mice ALS models have shown a marked reduction in the GLT-1 [
93]. Astrocytes that express mutated SOD1 fail to regulate the expression of glutamate receptor’s GluR2 subunit, and is present in motor neurons which leads to higher Ca
2+ levels and motor death [
94].
Parpura et al. via flash photolysis increased internal Ca
2+ in astrocytes to monitor Ca
2+ and glutamate levels that elicited slow inward currents. These electrophysiologically recorded signals showed that small variations in astrocytic Ca
2+, from 84 nM to 140 nM, elicited large glutamatergic currents in adjacent neurons. Therefore, the astrocytic glutamate release pathway is activated at normal levels of internal Ca
2+, as glutamate further elevates Ca
2+ in astrocytes to values surpassing 1.8 μM [
42]. When cytosolic Ca
2+ levels rise, mitochondria quickly absorb Ca
2+ to avoid Ca
2+ overload in the cytosol. But this excessive mitochondrial Ca
2+ uptake could lead to mitochondrial Ca
2+ overload and result in events like increase in reactive oxidative species (ROS), glutamate production inhibition of ATP synthesis mitochondrial permeability transition pore (mPTP) opening, the release of cytochrome C, activation of caspases, and apoptosis. The rise in Ca
2+ levels is transient in nature and occurs in the presence of disease pathologies involved in neurodegenerative diseases such as AD [
42,
95], PD [
96], multiple sclerosis [
97] and Huntington’s disease [
98].
Therefore, in such pathological conditions, astrocytes respond rapidly with numerous cellular adaptations, including morphological and functional rearrangements, gene and protein expression alterations, as well as changes in its secretome, which are collectively called reactive astrogliosis [
99]. The hallmark features of these reactive astrocytes are cell hypertrophy and up-regulation of Glial fibrillary acidic protein (GFAP) and vimentin (intermediate filaments). Along with these, they exhibit aberrant Ca
2+ signaling in a spatial and temporal dependent fashion, depending on the pathological condition [
100]. Since these Ca
2+-induced events are linked with glutamate exocytosis mediated by Ca
2+, their causal role in these types of neurodegenerative disorders needs to be emphasized.
2. Vacuolar ATPases (V-ATPases)
The V-ATPases are proton pumps with multiple subunits composed of a peripheral component V1 attached to an internal membrane-associated component V0 [
101]. The V0 is associated with the translocation of proteins while V1 is responsible for the hydrolysis of ATP [
102]. These pumps operate in conjunction with eight subunits that are present in V1 and six subunits present in V0 [
103]. The regulation of these pumps occurs in multiple processes such as the reversible dissociation of V1 and V0 subunits, disulfide bond formation, the altered proton transport to ATP hydrolysis ratio expressed as coupling efficiency, and modulation in the conductance of counterions [
104].
The presence of V-ATPases has been previously identified on the plasma membrane of astrocytes [
105] and presynaptic neurons; along with their vesicular membranes [
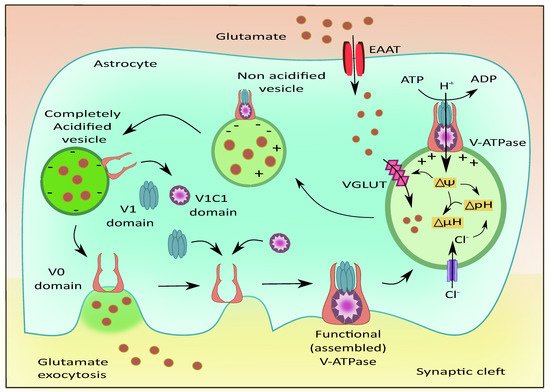
106]. V-ATPases play a role in maintaining acidic pH and the membrane potential to drive the filling of the vesicles with neurotransmitters as shown in
Figure 5 [
107]. They are also reported to exist in G1 and G2 isoforms where the G2 isoform is instrumental in maintaining the acidification of synaptic vesicles [
108]. The Voa1 and Voa2 components of V-ATPases were identified on the vesicles in PC12 cell lines [
109]. Interestingly, V-ATPases were recently known to have no direct contribution to the fusion of synaptic vesicles. They are released as V0, V1, and V1C1 components from the acidified vesicles [
110]. The V1 deficient synaptic vesicles bind to the plasma membrane and cause the recruitment of other components upon contact with luminal pH. This leads to endocytosis of vesicles that contain the fully assembled V-ATPases [
111]. This is followed by the recruitment of H+ ions into the vesicular lumen, the generation of membrane potential, and the filling of glutamate [
112].
Figure 5. Role of V-ATPases in vesicular glutamate filling and its exocytosis. The subunits of V-ATPases namely V0, V1, and V1C1 assemble to form a functional entity that generates the membrane potential and pH gradient necessary for the uptake of glutamate into the vesicles via VGLUT. Upon complete acidification of the vesicle, the V1 and V1C1 domains detach from the complex while the V0 domain facilitates membrane binding of the vesicle followed by glutamate exocytosis.
The VGLUTs are responsible for recruiting glutamate molecules into the synaptic vesicles [
113]. Montana et al. have reported the presence of VGLUTs in astrocytes and their role in astrocytic glutamate transmission [
35]. The V-ATPases were localized on the surface of astrocytes present in the hippocampus, and these pumps were responsible for the regulation of intracellular pH (pH
i) [
114]. The primary astrocytic cultures are mainly dependent on HCO3—independent mechanisms for maintaining the pH
i in comparison to cultured astrocytes [
115]. The process of neurotransmitter vesicular refilling mainly relies on the electrochemical gradient. This gradient is fulfilled by the V-ATPases and also by chloride ion channels by generating the required chemical gradient and membrane potential to facilitate this uptake [
116]. To a small extent, glutamate uptake is dependent on the chemical gradient, while optimum membrane potential is extremely necessary for the vesicular entry of glutamate. Interestingly, since glutamate itself is anionic, it acidifies the vesicles and activates the V-ATPases, enhancing the vesicular filling of glutamate via VGLUT [
117]. Another important factor for the optimum functioning of the V-ATPases is the ratio of ADP and ATP in astrocytes. Altered levels of ATP could affect the functioning of these pumps and prevent the development of the required proton gradient that would affect glutamate uptake into the vesicles thus affecting the release of glutamate [
118]. The mode of ATP release could be through a Ca
2+ dependent method—where the ATP is transported to the plasma membrane of astrocytes through secretory vesicles—and a Ca
2+ independent method—where astrocytic hemichannels and purinergic receptors, could release ATP [
119].
The blockade of astrocytic V-ATPase with bafilomycin A1, a V-ATPase inhibitor, showed alterations in the expression of TNF-α [
120] and low levels of glutamate release in astrocytes [
35]. Similarly, the administration of N-ethylmaleimide (NEM) reduced the pHi and incorporation of a more selective inhibitor of V-ATPases, 7-chloro-4-nitroben-2-oxa-1,3-diazole confirmed the vacuolar nature of these pumps [
121]. NEM appears to activate potassium ion antiport [
122] but the exact mechanism by which it reduces pH
i is not known.
The lysosomal degradation is executed by the hydrolytic enzymes, which are activated in acidic pH. The V-ATPase maintains this acidic pH by pumping protons into the lumen, by utilizing ATP [
32]. Lysosomal exocytotic release occurs much more slowly as compared to the release of neurotransmitters through vesicles [
123]. As the lysosomes contain ATP, lysosomal impairment prevents ATP-mediated Ca
2+ release, ultimately affecting astrocytic exocytosis governed by Ca
2+ stimulation [
124]. This provides evidence for the importance of lysosomal functioning in astrocytes.
The V-ATPases have been implicated in the pathogenesis of neurodegenerative diseases like AD and PD. As discussed earlier, the reversible translocation of V0 and V1 subunits are essential in the optimal functioning of the V-ATPases. The ATP6V1-A was found to be downregulated in the conditions of AD that impacted the neurotransmitter release from synaptic vesicles and prevented phosphorylation and phagosome formation thus worsening AD pathologies [
125]. ATP6AP2 is an important accessory protein that promotes neuronal growth in the CNS. Recently, splice variants of ATP6AP2 demonstrated defects in the acidification of lysosomes and progressed towards neuronal death. In in vitro systems, the ATP6AP2 deficits led to impaired V-ATPase assembly, thus affecting its function. The loss or mutations in Presenilin1 (PS1) have contributed to the pathologies of AD [
126]. This could be the reason why PS1 deletions showed impaired acidification of lysosomes and impaired autophagy, thus hampering the clearance of oligomers in AD [
127].
In the conditions of PD, lysosomal clearance of aggregates, such as α-synuclein, proteins with misfolded morphology, or debris, is essential which is regulated by V-ATPase activity [
128]. The mutations in the ATP6AP2 were correlated with progression towards parkinsonism in patients with X-linked parkinsonian syndrome [
129]. These studies demonstrate the importance of V-ATPases and their putative implication in neurodegenerative diseases, which is still underexplored.
This entry is adapted from the peer-reviewed paper 10.3390/cells11071139