The complex process of placental implantation and development affects trophoblast progenitors and uterine cells through the regulation of transcription factors, cytokines, adhesion receptors and their ligands. Differentiation of trophoblast precursors in the trophectoderm of early ontogenesis, caused by the transcription factors, such as CDX2, TEAD4, Eomes and GATA3, leads to the formation of cytotrophoblast and syncytiotrophoblast populations. The molecular mechanisms involved in placental formation inside the human body along with the specification and differentiation of trophoblast cell lines are, mostly due to the lack of suitable cell models, not sufficiently elucidated. This research is an evaluation of current technologies, which are used to study the behavior of human trophoblasts and other placental cells, as well as their ability to represent physiological conditions both in vivo and in vitro. An in vitro 3D model with a characteristic phenotype is of great benefit for the study of placental physiology. At the same time, it provides great support for future modeling of placental disease.
- trophoblast stem cells
- trophoblast invasion
- organoids
1. Primoculture Trophoblasts Monolayer Cells
2. Human Cancer Cell Lines
3. Trophoblast Stem Cells of the Blastocyst
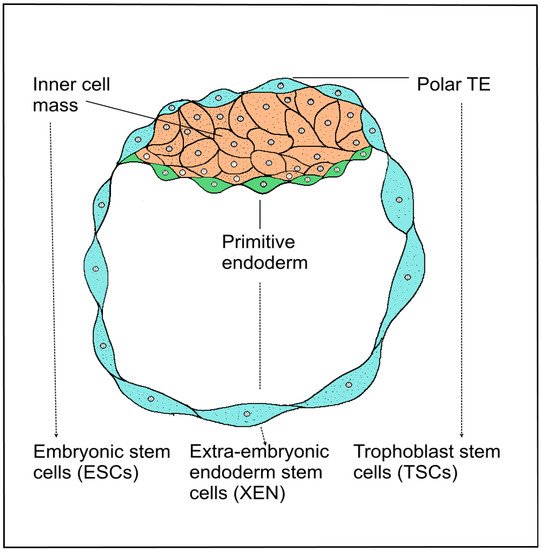
4. Induced Stem Cell Engineering Cell Fate
5. Trophoblast Organoids and Spheroids as Placental Model
5.1. Placental Tissue Culture
5.2. Organoids Mimic EVT
5.3. Spheroids of Placenta-Derived Mesenchymal Stem Cells
5.4. Characteristics Phenotype of Trophoblast Organoids
This entry is adapted from the peer-reviewed paper 10.3390/biomedicines10040904
References
- Kliman, H.J. Uteroplacental blood flow. The story of decidualization, menstruation, and trophoblast invasion. Am. J. Pathol. 2000, 157, 1759–1768.
- Okae, H.; Toh, H.; Sato, T.; Hiura, H.; Takahashi, S.; Shirane, K. Derivation of human trophoblast stem cells. Cell Stem Cell 2018, 22, 50–63.
- Ringler, G.E.; Strauss, J.F., III. In vitro systems for the study of human placental endocrine function. Endocr. Rev. 1990, 11, 105–123.
- Bangma, J.; Szilagyi, J.; Blake, B.E.; Plazas, C.; Kepper, S.; Fenton, S.E. An assessment of serum-dependent impacts on intracellular accumulation and genomic response of per-and polyfluoroalkyl substances in a placental trophoblast model. Environ. Toxicol. 2020, 35, 1395–1405.
- Heaton, S.J.; Eady, J.J.; Parker, M.L.; Gotts, K.L.; Dainty, J.R. The use of BeWo cells as an in vitro model for placental iron transport. Am. J. Physiol.-Cell Physiol. 2008, 295, 1445–1453.
- Abou-Kheir, W.; Barrak, J.; Hadadeh, O.; Daoud, G. HTR-8/SVneo cell line contains a mixed population of cells. Placenta 2017, 50, 1–7.
- Abbas, Y.; Turco, M.Y.; Burton, G.J.; Moffett, A. Investigation of human trophoblast invasion in vitro. Hum. Reprod. Update 2020, 26, 501–513.
- Jingting, C.; Yangde, Z.; Yi, Z.; Huining, L.; Rong, Y. Heparanase expression correlates with metastatic capability in human choriocarcinoma. Gynecol. Oncol. 2007, 107, 22–29.
- Apps, R.; Murphy, S.P.; Fernando, R.; Gardner, L.; Ahad, T.; Moffett, A. Human leucocyte antigen (HLA) expression of primary trophoblast cells and placental cell lines, determined using single antigen beads to characterize allotype specificities of anti-HLA antibodies. Immunology 2009, 127, 26–39.
- Apps, R.; Sharkey, A.; Gardner, L.; Male, V.; Trotter, M.; Miller, N.; Moffett, A. Genome-wide expression profile of first trimester villous and extravillous human trophoblast cells. Placenta 2011, 32, 33–43.
- Manaster, I.; Goldman-Wohl, D.; Greenfield, C.; Nachmani, D.; Tsukerman, P.; Hamani, Y. MiRNA-mediated control of HLA-G expression and function. PLoS ONE 2012, 7, e33395.
- Kubaczka, C.; Kaiser, F.; Schorle, H. Breaking the first lineage barrier–many roads to trophoblast stem cell fate. Placenta 2017, 60, 52–56.
- Castel, G.; Meistermann, D.; Bretin, B.; Firmin, J.; Blin, J.; Loubersac, S. Induction of Human Trophoblast Stem Cells from Somatic Cells and Pluripotent Stem Cells. Cell Rep. 2020, 33, 108419.
- Haider, S.; Meinhardt, G.; Saleh, L.; Kunihs, V.; Gamperl, M.; Kaindl, U. Self-renewing trophoblast organoids recapitulate the developmental program of the early human placenta. Stem Cell Rep. 2018, 11, 537–551.
- Genbacev, O.D.; Prakobphol, A.; Foulk, R.A.; Krtolica, A.R.; Ilic, D. Trophoblast L-selectin-mediated adhesion at the maternal-fetal interface. Science 2003, 299, 405–408.
- Zdravkovic, T.; Nazor, K.L.; Larocque, N.; Gormley, M.; Donne, M.; Giritharan, G.; Fisher, S.J. Human stem cells from single blastomeres reveal pathways of embryonic or trophoblast fate specification. Development 2015, 142, 4010–4025.
- Yang, Y.; Parker, G.C.; Puscheck, E.E.; Rappolee, D.A. Direct reprogramming to multipotent trophoblast stem cells, and is pluripotency needed for regenerative medicine either? Stem Cell Investig. 2016, 3, 24.
- Deglincerti, A.; Croft, G.F.; Pietila, L.N.; Zernicka-Goetz, M.; Siggia, E.D.; Brivanlou, A.H. Self-organization of the in vitro attached human embryo. Nature 2016, 533, 251–254.
- Roberts, R.M.; Fisher, S.J. Trophoblast stem cells. Biol. Reprod. 2011, 84, 412–421.
- Chen, Y.; Wang, K.; Gong, Y.G.; Khoo, S.K.; Leach, R. Roles of CDX2 and EOMES in human induced trophoblast progenitor cells. Biochem. Biophys. Res. Commun. 2013, 431, 197–202.
- Lee, C.Q.; Gardner, L.; Turco, M.; Zhao, N.; Murray, M.J.; Coleman, N. What is trophoblast? A combination of criteria define human first-trimester trophoblast. Stem Cell Rep. 2016, 6, 257–272.
- Turco, M.Y.; Moffett, A. Development of the human placenta. Development 2019, 146, 163428.
- Miller, R.K.; Genbacev, O.; Turner, M.A.; Aplin, J.D.; Caniggia, I. Human placental explants in culture: Approaches and assessments. Placenta 2005, 26, 439–448.
- Turco, M.Y.; Gardner, L.; Kay, R.G.; Hamilton, R.S.; Prater, M.; Hollinshead, M.S. Trophoblast organoids as a model for maternal–fetal interactions during human placentation. Nature 2018, 564, 263–267.
- Kretzschmar, K.; Clevers, H. Organoids: Modeling development and the stem cell niche in a dish. Dev. Cell 2016, 38, 590–600.
- Lee, C.Q.; Turco, M.Y.; Gardner, L.; Simons, B.D.; Hemberger, M.; Moffett, A. Integrin α2 marks a niche of trophoblast progenitor cells in first trimester human placenta. Development 2018, 145, 16.
- Horii, M.; Touma, O.; Bui, T.; Parast, M.M. Modeling human trophoblast, the placental epithelium at the maternal fetal interface. Reproduction 2020, 160, R1–R11.
- Dong, C.; Beltcheva, M.; Gontarz, P.; Zhang, B.; Popli, P.; Fischer, L.A.; Theunissen, T.W. Derivation of trophoblast stem cells from naïve human pluripotent stem cells. eLife 2020, 9, 52504.
- Wooding, F.B.P. The synepitheliochorial placenta of ruminants: Binucleate cell fusions and hormone production. Placenta 1992, 13, 101–113.
- Kadyrov, M.; Garnier, Y.; Gantert, M.; Kramer, B.W.; Kaufmann, P.; Huppertz, B. Cytokeratin antibodies as differential markers of trophoblast and fetomaternal syncytial plaques in the sheep placentome. Placenta 2007, 28, 1107–1109.
- Highet, A.R.; Zhang, V.J. Use of Matrigel in culture affects cell phenotype and gene expression in the first trimester trophoblast cell line HTR8/SVneo. Placenta 2012, 33, 586–588.
- Bárcia, R.N.; Santos, J.M.; Teixeira, M.; Filipe, M.; Pereira, A.R.S.; Ministro, A. Umbilical cord tissue–derived mesenchymal stromal cells maintain immunomodulatory and angiogenic potencies after cryopreservation and subsequent thawing. Cytotherapy 2017, 19, 360–370.
- Parolini, O.; Alviano, F.; Bagnara, G.P.; Bilic, G.; Bühring, H.J.; Evangelista, M. Concise review: Isolation and characterization of cells from human term placenta: Outcome of the first international Workshop on Placenta Derived Stem Cells. Stem Cells 2008, 26, 300–311.
- Bačenková, D.; Rosocha, J.; Tóthová, T.; Rosocha, L.; Šarisský, M. Isolation and basic characterization of human term amnion and chorion mesenchymal stromal cells. Cytotherapy 2011, 13, 1047–1056.
- Bačenková, D.; Trebuňová, M.; Zachar, L.; Hudák, R.; Ižaríková, G.; Šurínová, K.; Živčák, J. Analysis of same selected immunomodulatory properties of chorionic mesenchymal stem cells. Appl. Sci. 2020, 10, 9040.
- Janockova, J.; Slovinska, L.; Harvanova, D.; Spakova, T.; Rosocha, J. New therapeutic approaches of mesenchymal stem cells-derived exosomes. J. Biomed. Sci. 2021, 28, 39.
- Lankford, L.; Chen, Y.J.; Saenz, Z.; Kumar, P.; Long, C.; Farmer, D.; Wang, A. Manufacture and preparation of human placenta-derived mesenchymal stromal cells for local tissue delivery. Cytotherapy 2017, 19, 680–688.
- Rettinger, C.L.; Fourcaudot, A.B.; Hong, S.J.; Mustoe, T.A.; Hale, R.G.; Leung, K.P. In vitro characterization of scaffold-free three-dimensional mesenchymal stem cell aggregates. Cell Tissue Res. 2014, 358, 395–405.
- Miceli, V.; Pampalone, M.; Vella, S.; Carreca, A.P.; Amico, G.; Conaldi, P.G. Comparison of immunosuppressive and angiogenic properties of human amnion-derived mesenchymal stem cells between 2D and 3D culture systems. Stem Cells Int. 2019, 2019, 7486279.
- Vikartovska, Z.; Humenik, F.; Maloveska, M.; Farbakova, J.; Hornakova, L.; Murgoci, A.N.; Cizkova, D. Adult Stem Cells Based Therapies in Veterinary Medicine. Arch. Vet. Sci. Med. 2020, 3, 40–50.
- Trebuňova, M.; Gromošová, S.; Bačenková, D.; Rosocha, J.; Živčák, J. Biocompatibility of the human mesenchymal stem cells with bovine bone tissue at the cellular level in vitro. Lékař A Tech. Clin. Technol. 2018, 48, 59–65.
- Morrish, D.W.; Bhardwaj, D.; Paras, M.T. Transforming growth factor β1 inhibits placental differentiation and human chorionic gonadotropin and human placental lactogen secretion. Endocrinology 1991, 129, 22–26.
- Knöfler, M.; Pollheimer, J. IFPA Award in Placentology lecture: Molecular regulation of human trophoblast invasion. Placenta 2012, 33, 55–62.
- Chawengsaksophak, K.; de Graaff, W.; Rossant, J.; Deschamps, J.; Beck, F. Cdx2 is essential for axial elongation in mouse development. Proc. Natl. Acad. Sci. USA 2004, 101, 7641–7645.
- Crum, C.P.; McKeon, F.D. p63 in epithelial survival, germ cell surveillance, and neoplasia. Annu. Rev. Pathol. Mech. Dis. 2010, 5, 349–371.
- Li, Y.; Moretto-Zita, M.; Leon-Garcia, S.; Parast, M.M. p63 inhibits extravillous trophoblast migration and maintains cells in a cytotrophoblast stem cell-like state. Am. J. Pathol. 2014, 184, 3332–3343.
- Biadasiewicz, K.; Sonderegger, S.; Haslinger, P.; Haider, S.; Saleh, L.; Fiala, C. Transcription factor AP-2α promotes EGF-dependent invasion of human trophoblast. Endocrinology 2011, 152, 1458–1469.
- Plessl, K.; Haider, S.; Fiala, C.; Pollheimer, J.; Knöfler, M. Expression pattern and function of Notch2 in different subtypes of first trimester cytotrophoblast. Placenta 2015, 36, 365–371.
