Coccidioidomycosis, also known as Valley fever, is an endemic fungal infection commonly found in the southwestern parts of the United States. However, the disease has seen an increase in both in its area of residency and its prevalence. This entry compiles some of the latest information on the epidemiology, current and in-development pharmaceutical approaches to treat the disease, trends and projections, diagnostic concerns, and the overlapping dynamics of coccidioidomycosis and COVID-19, including in special populations. This entry provides an overview of the current diagnostic and therapeutic strategies and identifies areas of future development.
- antifungal agents
- coccidioidomycosis
- Coccidioides spp.
1. Introduction
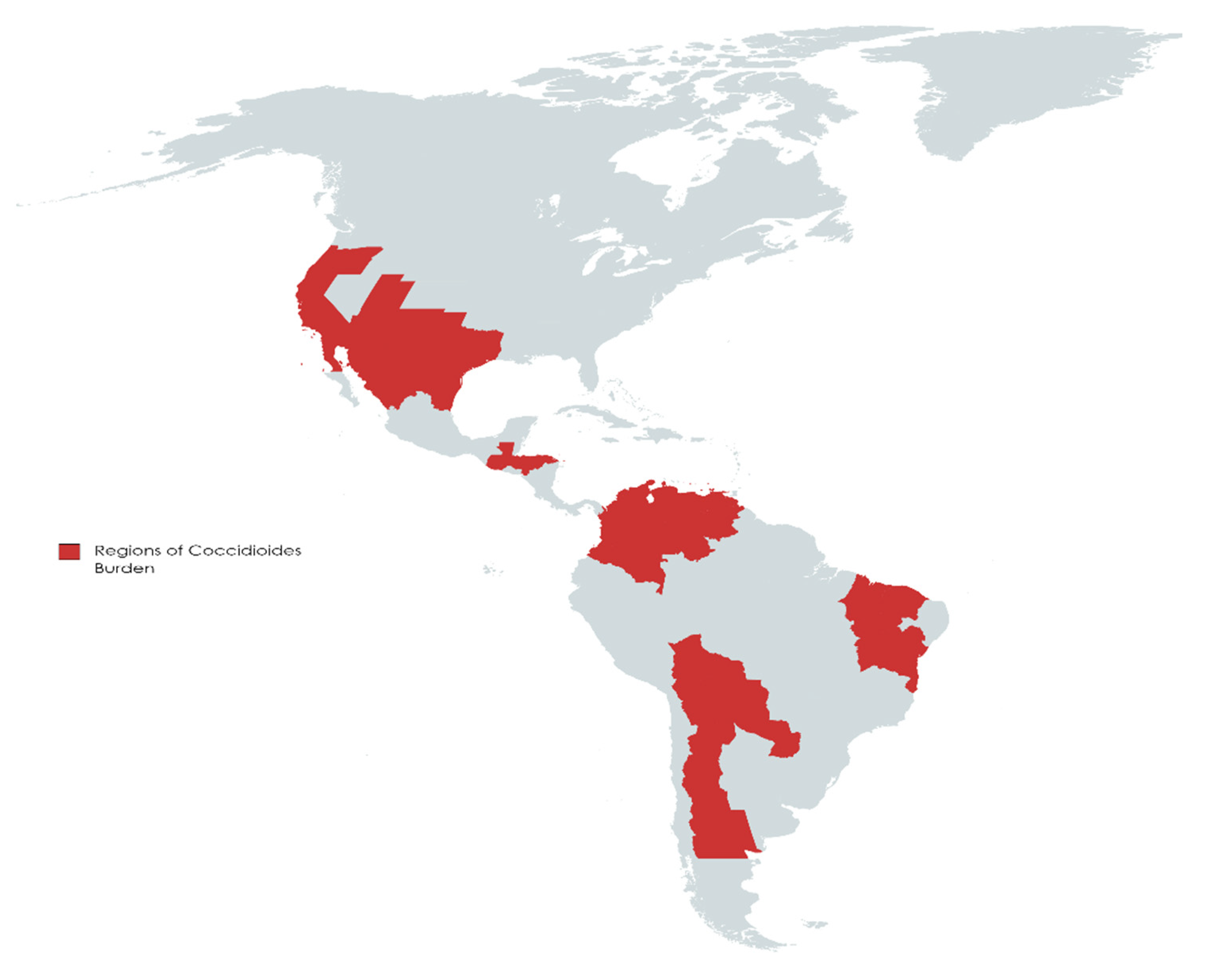
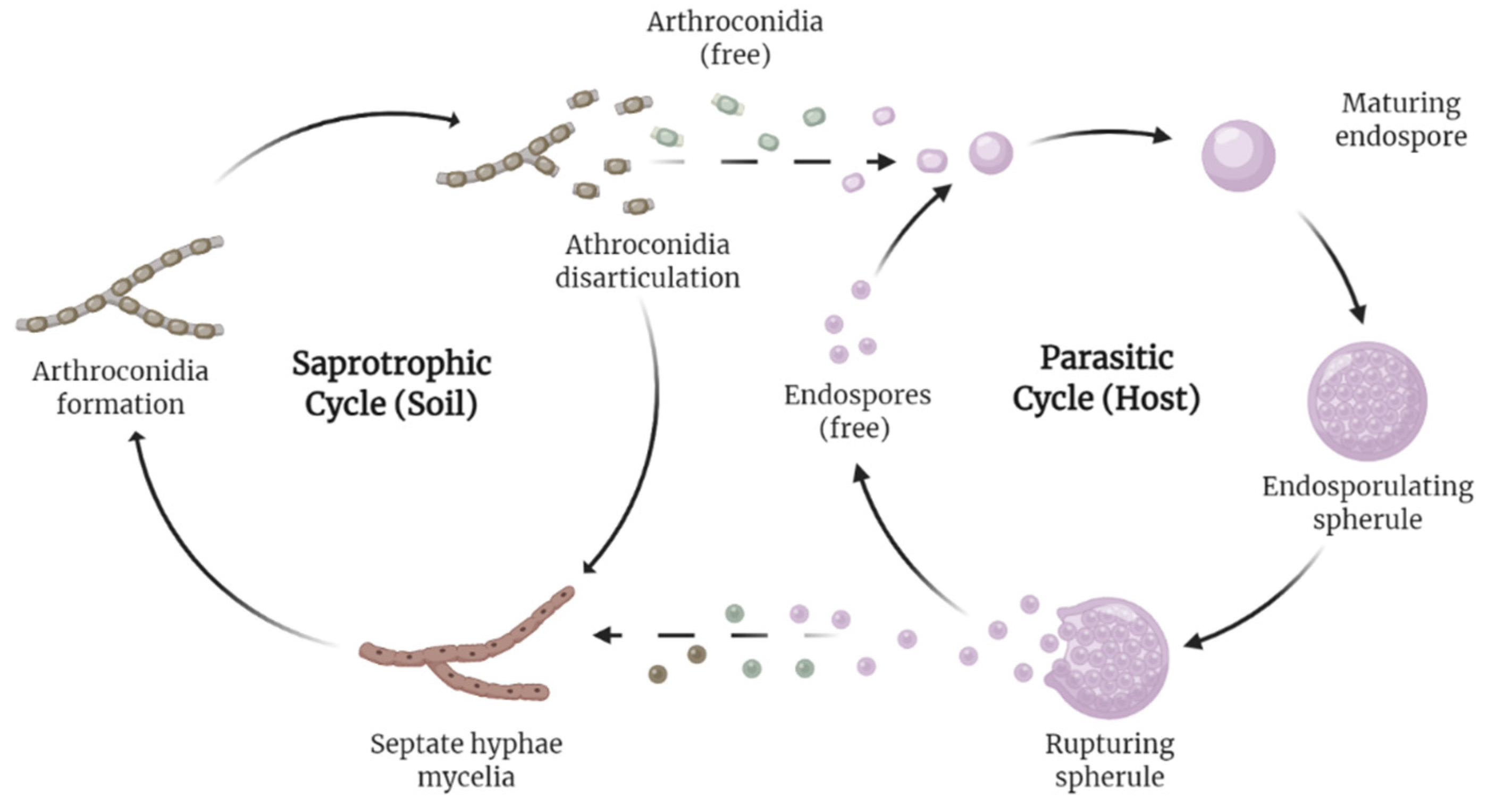
2. Burden and Projections
3. Diagnosis
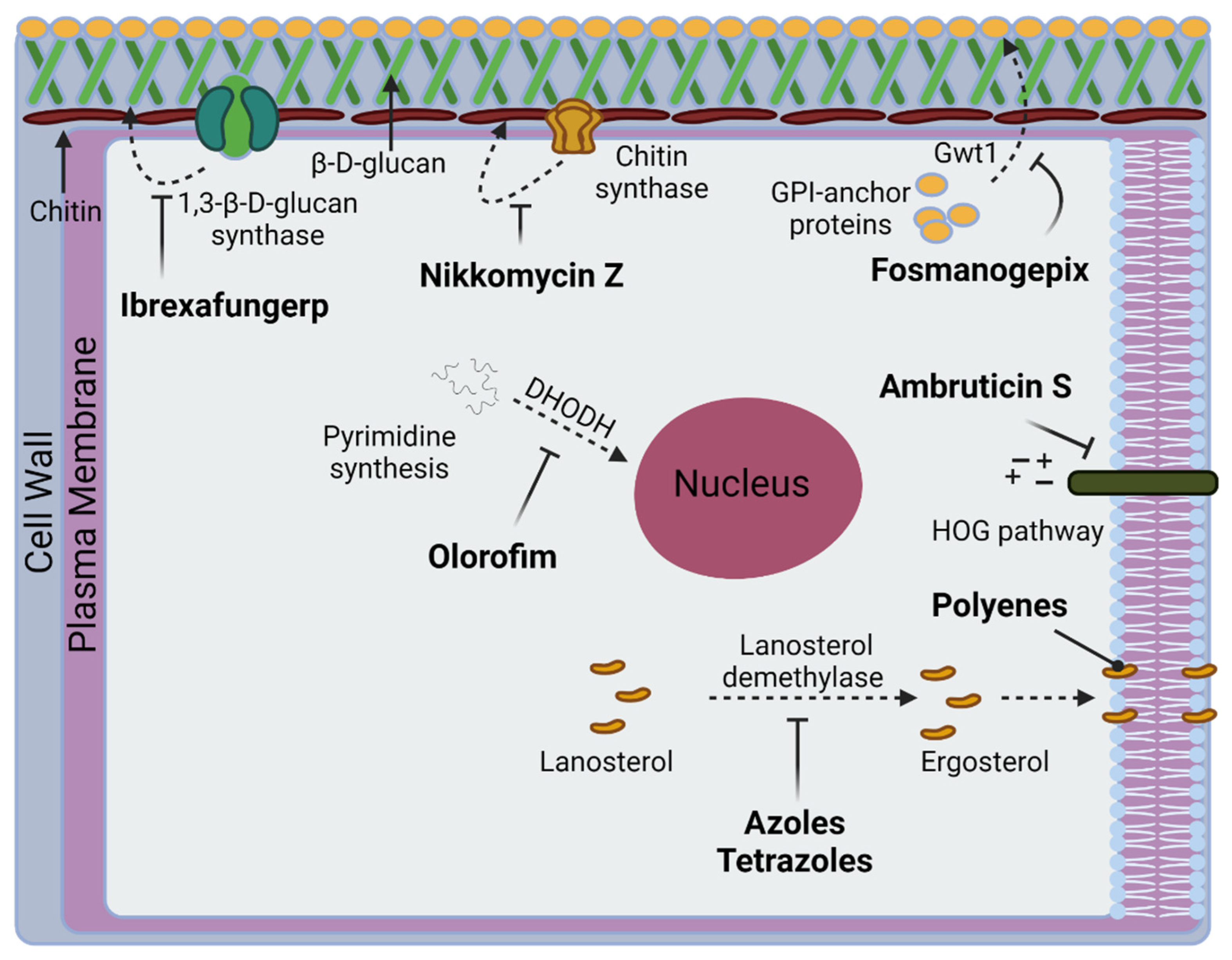
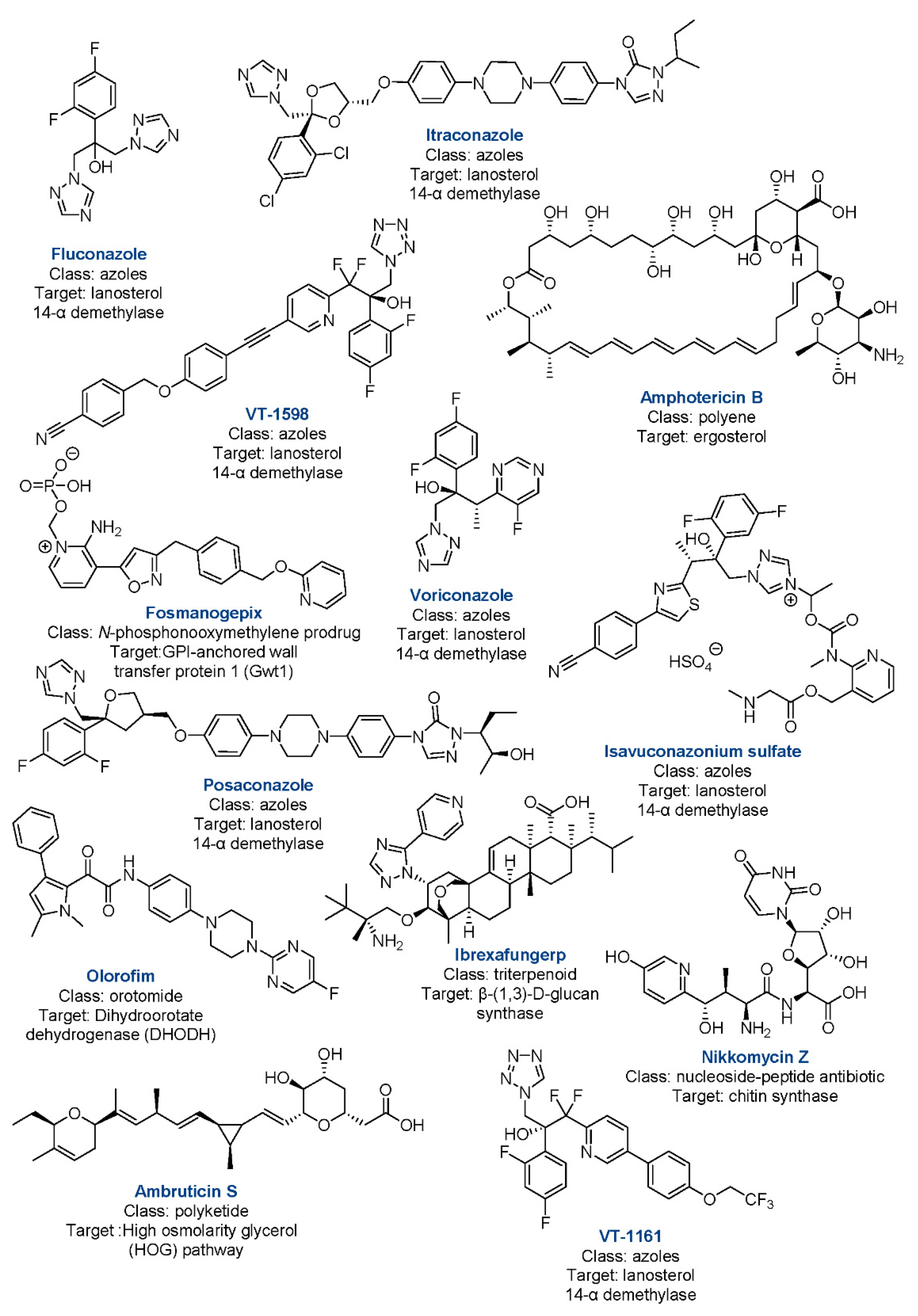
4. Currently Approved Drugs
This entry is adapted from the peer-reviewed paper 10.3390/jof8040413
References
- Brown, J.; Benedict, K.; Park, B.J.; Thompson, G.R., 3rd. Coccidioidomycosis: Epidemiology. Clin. Epidemiol. 2013, 5, 185–197.
- Park, J.; Chaffee, A.W.; Harrigan, R.J.; Schoenberg, F.P. A non-parametric Hawkes model of the spread of Ebola in west Africa. J. Appl. Stat. 2020, 49, 621–637.
- Posadas, A. Un nuevo caso de micosis fungoidea con posrospemias. Ann. Cir. Med. Argent 1892, 15, 585–597.
- Fisher, M.C.; Koenig, G.L.; White, T.J.; Taylor, J.W. Molecular and phenotypic description of Coccidioides posadasii sp. nov., previously recognized as the non-California population of Coccidioides immitis. Mycologia 2002, 94, 73–84.
- Bajwa, A.K.; Rongkavilit, C. Update on Coccidioidomycosis in the United States and Beyond. Glob. Pediatr. Health 2020, 7, 2333794x20969282.
- Laniado-Laborín, R.; Arathoon, E.G.; Canteros, C.; Muñiz-Salazar, R.; Rendon, A. Coccidioidomycosis in Latin America. Med. Mycol. 2019, 57, S46–S55.
- Morais, J.; Borges, M.C.M.; Cavalcante, L.; Motoyama, P.V.P.; Libório, M.P.; Távora, L.G.F. Coccidioidomycosis in a reference center in Northeast Brazil: Clinical/epidemiological profile and most common radiological findings. Rev. Soc. Bras. Med. Trop. 2020, 53, e20200249.
- Lockhart, S.R.; Toda, M.; Benedict, K.; Caceres, D.H.; Litvintseva, A.P. Endemic and Other Dimorphic Mycoses in The Americas. J. Fungi 2021, 7, 151.
- Huppert, M.; Sun, S.H.; Harrison, J.L. Morphogenesis throughout saprobic and parasitic cycles of Coccidioides immitis. Mycopathologia 1982, 78, 107–122.
- Nguyen, C.; Barker, B.M.; Hoover, S.; Nix, D.E.; Ampel, N.M.; Frelinger, J.A.; Orbach, M.J.; Galgiani, J.N. Recent advances in our understanding of the environmental, epidemiological, immunological, and clinical dimensions of coccidioidomycosis. Clin. Microbiol. Rev. 2013, 26, 505–525.
- Sharpton, T.J.; Stajich, J.E.; Rounsley, S.D.; Gardner, M.J.; Wortman, J.R.; Jordar, V.S.; Maiti, R.; Kodira, C.D.; Neafsey, D.E.; Zeng, Q.; et al. Comparative genomic analyses of the human fungal pathogens Coccidioides and their relatives. Genome Res. 2009, 19, 1722–1731.
- Taylor, J.W.; Barker, B.M. The endozoan, small-mammal reservoir hypothesis and the life cycle of Coccidioides species. Med. Mycol. 2019, 57, S16–S20.
- Shubitz, L.E.; Butkiewicz, C.D.; Dial, S.M.; Lindan, C.P. Incidence of coccidioides infection among dogs residing in a region in which the organism is endemic. J. Am. Vet. Med. Assoc. 2005, 226, 1846–1850.
- Shubitz, L.F. Comparative aspects of coccidioidomycosis in animals and humans. Ann. N. Y. Acad. Sci. 2007, 1111, 395–403.
- Wilson, L.; Ting, J.; Lin, H.; Shah, R.; MacLean, M.; Peterson, M.W.; Stockamp, N.; Libke, R.; Brown, P. The Rise of Valley Fever: Prevalence and Cost Burden of Coccidioidomycosis Infection in California. Int. J. Environ. Res. Public Health 2019, 16, 1113.
- Increase in reported coccidioidomycosis—United States, 1998–2011. MMWR Morb. Mortal. Wkly. Rep. 2013, 62, 217–221.
- Oltean, H.N.; Springer, M.; Bowers, J.R.; Barnes, R.; Reid, G.; Valentine, M.; Engelthaler, D.M.; Toda, M.; McCotter, O.Z. Suspected Locally Acquired Coccidioidomycosis in Human, Spokane, Washington, USA. Emerg. Infect. Dis. 2020, 26, 606–609.
- Johnson, S.M.; Carlson, E.L.; Fisher, F.S.; Pappagianis, D. Demonstration of Coccidioides immitis and Coccidioides posadasii DNA in soil samples collected from Dinosaur National Monument, Utah. Med. Mycol. 2014, 52, 610–617.
- Yoo, S.D.; Lusiba, J.K.; Lukande, R.; Shin, K. Disseminated Coccidioidomycosis in Africa. Eur. J. Case Rep. Intern. Med. 2020, 7, 001659.
- Hernandez, H.; Erives, V.H.; Martinez, L.R. Coccidioidomycosis: Epidemiology, Fungal Pathogenesis, and Therapeutic Development. Curr. Trop. Med. Rep. 2019, 6, 132–144.
- Huang, J.Y.; Bristow, B.; Shafir, S.; Sorvillo, F. Coccidioidomycosis-associated Deaths, United States, 1990–2008. Emerg. Infect. Dis. 2012, 18, 1723–1728.
- Valdivia, L.; Nix, D.; Wright, M.; Lindberg, E.; Fagan, T.; Lieberman, D.; Stoffer, T.; Ampel, N.M.; Galgiani, J.N. Coccidioidomycosis as a common cause of community-acquired pneumonia. Emerg. Infect. Dis. 2006, 12, 958–962.
- Johnson, R.H.; Sharma, R.; Kuran, R.; Fong, I.; Heidari, A. Coccidioidomycosis: A review. J. Investig. Med. 2021, 69, 316–323.
- Galgiani, J.N.; Ampel, N.M.; Blair, J.E.; Catanzaro, A.; Johnson, R.H.; Stevens, D.A.; Williams, P.L. Coccidioidomycosis. Clin. Infect. Dis. 2005, 41, 1217–1223.
- Johnson, R.; Caldwell, J.; Welch, G.; Einstein, H. The great coccidioidomycosis epidemic: Clinical features. In Proceedings of the Coccidioidomycosis: Fifth International Conference; National Foundation for Infectious Diseases: Washington, DC, USA, 1996.
- Odio, C.D.; Marciano, B.E.; Galgiani, J.N.; Holland, S.M. Risk Factors for Disseminated Coccidioidomycosis, United States. Emerg. Infect. Dis. 2017, 23, 308–311.
- Goldstein, E.J.C.; Johnson, R.H.; Einstein, H.E. Coccidioidal Meningitis. Clin. Infect. Dis. 2006, 42, 103–107.
- McCotter, O.Z.; Benedict, K.; Engelthaler, D.M.; Komatsu, K.; Lucas, K.D.; Mohle-Boetani, J.C.; Oltean, H.; Vugia, D.; Chiller, T.M.; Sondermeyer Cooksey, G.L.; et al. Update on the Epidemiology of coccidioidomycosis in the United States. Med. Mycol. 2019, 57, S30–S40.
- Gorris, M.E.; Neumann, J.E.; Kinney, P.L.; Sheahan, M.; Sarofim, M.C. Economic Valuation of Coccidioidomycosis (Valley Fever) Projections in the United States in Response to Climate Change. Weather Clim. Soc. 2021, 13, 107–123.
- Gnat, S.; Łagowski, D.; Nowakiewicz, A.; Dyląg, M. A global view on fungal infections in humans and animals: Infections caused by dimorphic fungi and dermatophytoses. J. Appl. Microbiol. 2021, 131, 2688–2704.
- Casadevall, A.; Kontoyiannis, D.P.; Robert, V. On the Emergence of Candida auris: Climate Change, Azoles, Swamps, and Birds. mBio 2019, 10, e01397-19.
- Ashraf, N.; Kubat, R.C.; Poplin, V.; Adenis, A.A.; Denning, D.W.; Wright, L.; McCotter, O.; Schwartz, I.S.; Jackson, B.R.; Chiller, T.; et al. Re-drawing the Maps for Endemic Mycoses. Mycopathologia 2020, 185, 843–865.
- Pearson, D.; Ebisu, K.; Wu, X.; Basu, R. A Review of Coccidioidomycosis in California: Exploring the Intersection of Land Use, Population Movement, and Climate Change. Epidemiol. Rev. 2019, 41, 145–157.
- Matlock, M.; Hopfer, S.; Ogunseitan, O.A. Communicating Risk for a Climate-Sensitive Disease: A Case Study of Valley Fever in Central California. Int. J. Environ. Res. Public Health 2019, 16, 3254.
- Shiu, J.; Thai, M.; Elsensohn, A.N.; Nguyen, N.Q.; Lin, K.Y.; Cassarino, D.S. A case series of primary cutaneous coccidioidomycosis after a record-breaking rainy season. JAAD Case Rep. 2018, 4, 412–414.
- Zender, C.S.; Talamantes, J. Climate controls on valley fever incidence in Kern County, California. Int. J. Biometeorol. 2006, 50, 174–182.
- Coates, S.J.; Fox, L.P. Disseminated coccidioidomycosis as a harbinger of climate change. JAAD Case Rep. 2018, 4, 424–425.
- Gorris, M.E.; Treseder, K.K.; Zender, C.S.; Randerson, J.T. Expansion of Coccidioidomycosis Endemic Regions in the United States in Response to Climate Change. Geohealth 2019, 3, 308–327.
- Wang, J.; Harrigan, R.J.; Schoenberg, F.P. Point Process Models for the Spread of Coccidioidomycosis in California. Infect. Dis. Rep. 2021, 13, 52.
- Mitchell, W.; Bhatia, R.; Zebardast, N. Retrospective cross-sectional analysis of the changes in marijuana use in the USA, 2005–2018. BMJ Open 2020, 10, e037905.
- McHardy, I.; Romanelli, A.; Harris, L.J.; Opp, G.; Gaudino, R.; Torres, A.; Polage, C.R.; Tuscano, J.M.; Thompson, G.R., 3rd. Infectious risks associated with medicinal Cannabis: Potential implications for immunocompromised patients? J. Infect. 2018, 76, 500–501.
- Shapiro Bb Md, M.P.H.; Hedrick, R.; Vanle, B.C.; Becker, C.A.; Nguyen, C.; Underhill, D.M.; Morgan, M.A.; Kopple, J.D.; Danovitch, I.; IsHak, W.W. Cryptococcal meningitis in a daily cannabis smoker without evidence of immunodeficiency. BMJ Case Rep. 2018, 2018, bcr-2017.
- Akram, S.M.; Koirala, J. Coccidioidomycosis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021.
- Balderas-Sosa, E.Y.; De la Torre, J.L.; Soualhi, A.; Leyva-Moraga, F.A.; Leyva-Moraga, F.; Leyva-Moraga, E. Coccidioidomycosis mimicking testicular cancer: A case report. Andrologia 2021, 53, e14151.
- Huang, J.; Cano, E.J.; Shweta, F.; Shah, A.S.; Schuetz, A.N.; Bois, M.; Gurram, P.R. Infected Aneurysm of the Native Aorta due to Coccidioides posadasii. Open Forum Infect. Dis. 2021, 8, ofab266.
- Nasrawi, F.; Heidari, A.; Aljashamy, T.; Mangat, N.; Bhaika, J.; Kaur, S.; Kuran, R.; Johnson, R. Disseminated Coccidioidomycosis Presenting as Polyarticular Septic Arthritis: A Case Report. J. Investig. Med. High Impact Case Rep. 2020, 8, 2324709620974894.
- Converse, C.; Dey, A.; Decker, S.; Arabian, S.; Neeki, M. Coccidioidomycosis of the Vocal Cords Presenting in Sepsis: A Case Report and Literature Review. Case Rep. Crit. Care 2020, 2020, 8025391.
- Aduroja, O.; Okudo, J.; Padilla, A. Disseminated Coccidioidomycosis Presenting as Septic Shock with Multiorgan Failure. Case Rep. Infect. Dis. 2021, 2021, 8837493.
- Pu, J.; Donovan, F.M.; Ellingson, K.; Leroy, G.; Stone, J.; Bedrick, E.; Galgiani, J.N. Clinician Practice Patterns that Result in the Diagnosis of Coccidioidomycosis Before or During Hospitalization. Clin. Infect. Dis. 2020, 73, e1587–e1593.
- Durkin, M.; Connolly, P.; Kuberski, T.; Myers, R.; Kubak, B.M.; Bruckner, D.; Pegues, D.; Wheat, L.J. Diagnosis of Coccidioidomycosis with Use of the Coccidioides Antigen Enzyme Immunoassay. Clin. Infect. Dis. 2008, 47, e69–e73.
- Saubolle, M.A. Laboratory Aspects in the Diagnosis of Coccidioidomycosis. Ann. N. Y. Acad. Sci. 2007, 1111, 301–314.
- Kassis, C.; Durkin, M.; Holbrook, E.; Myers, R.; Wheat, L. Advances in Diagnosis of Progressive Pulmonary and Disseminated Coccidioidomycosis. Clin. Infect. Dis. 2020, 72, 968–975.
- Galgiani, J.N.; Ampel, N.M.; Blair, J.E.; Catanzaro, A.; Geertsma, F.; Hoover, S.E.; Johnson, R.H.; Kusne, S.; Lisse, J.; MacDonald, J.D.; et al. 2016 Infectious Diseases Society of America (IDSA) Clinical Practice Guideline for the Treatment of Coccidioidomycosis. Clin. Infect. Dis. 2016, 63, e112–e146.
- Greiner, B.; Essex, R.; Wheeler, D. An analysis of research quality underlying IDSA clinical practice guidelines: A cross-sectional study. J. Osteopath. Med. 2021, 121, 319–323.
- DIFLUCAN (Fluconazole). New York, New York: Pfizer. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/019949s051lbl.pdf (accessed on 24 February 2022).
- Amphotericin, B. Big Flats, NY: X-Gen Pharmaceuticals, Inc. Available online: http://xgenpharmadjb.com/wp-content/uploads/sites/21/2021/12/ampho.pdf. (accessed on 24 February 2022).
- SPORANOX (Itraconazole). Raritan, NJ: Ortho-McNeil-Janssen Pharmaceuticals, Inc. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/020083s063lbl.pdf (accessed on 24 February 2022).
- Thompson, G.R., III; Lewis, J.S., II; Nix, D.E.; Patterson, T.F. Current Concepts and Future Directions in the Pharmacology and Treatment of Coccidioidomycosis. Med. Mycol. 2019, 57, S76–S84.
- Bercovitch, R.S.; Catanzaro, A.; Schwartz, B.S.; Pappagianis, D.; Watts, D.H.; Ampel, N.M. Coccidioidomycosis During Pregnancy: A Review and Recommendations for Management. Clin. Infect. Dis. 2011, 53, 363–368.
- Davis, M.R.; Nguyen, M.-V.H.; Donnelley, M.A.; Thompson, G.R., III. Tolerability of long-term fluconazole therapy. J. Antimicrob. Chemother. 2018, 74, 768–771.
- Thompson, G.R.; Barker, B.M.; Wiederhold, N.P. Large-Scale Evaluation of In Vitro Amphotericin B, Triazole, and Echinocandin Activity against Coccidioides Species from U.S. Institutions. Antimicrob. Agents Chemother. 2017, 61, e02634-02616.
- Decembrino, N.; Perruccio, K.; Zecca, M.; Colombini, A.; Calore, E.; Muggeo, P.; Soncini, E.; Comelli, A.; Molinaro, M.; Goffredo, B.M.; et al. A Case Series and Literature Review of Isavuconazole Use in Pediatric Patients with Hemato-oncologic Diseases and Hematopoietic Stem Cell Transplantation. Antimicrob. Agents Chemother. 2020, 64, e01783-01719.
- Thompson, G.R., 3rd; Wiederhold, N.P. Isavuconazole: A comprehensive review of spectrum of activity of a new triazole. Mycopathologia 2010, 170, 291–313.
- Heidari, A.; Quinlan, M.; Benjamin, D.J.; Laurence, B.; Mu, A.; Ngai, T.; Hoffman, W.J.; Cohen, S.H.; McHardy, I.; Johnson, R.; et al. Isavuconazole in the Treatment of Coccidioidal Meningitis. Antimicrob. Agents Chemother. 2019, 63, e02232-02218.
- Kovanda, L.L.; Sass, G.; Martinez, M.; Clemons, K.V.; Nazik, H.; Kitt, T.M.; Wiederhold, N.; Hope, W.W.; Stevens, D.A. Efficacy and Associated Drug Exposures of Isavuconazole and Fluconazole in an Experimental Model of Coccidioidomycosis. Antimicrob. Agents Chemother. 2021, 65, e02344-02320.
