Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Protein kinases (PTKs) are enzymes that regulate the biological activity of proteins by phosphorylation of certain amino acid residues. This reaction causes a conformational change from an inactive to an active form of the protein, which is one of the most important regulatory mechanisms of the cell cycle and transduction of external signals. Dysregulation of protein kinases activity is implicated in the processes of carcinogenesis and the progression of various solid cancers. Therefore, protein kinases are prime targets for the development of selective anticancer drugs.
- Protein kinases
- Anticancer agents
- Small molecule inhibitors
1. Tyrosine Kinase (TK) Inhibitors
Tyrosine kinases (RTKs) are enzymes that selectively phosphorylate the hydroxyl groups of a tyrosine residue in different proteins with adenosine triphosphate (ATP) as the source of phosphate. They have a share in the regulation of the most fundamental cellular processes, such as growth, differentiation, proliferation, survival, migration and metabolism of cells or programed cell death in response to extracellular and intracellular stimuli [1]. There are two types of tyrosine kinases, namely receptor tyrosine kinases (RTKs) and nonreceptor tyrosine kinases (NRTKs) [2]. A lot of RTKs and NRTKs are associated with cancers, thus a significant number of tyrosine kinase inhibitors (TKIs) are currently in clinical development. Since 2011, the FDA approved eleven new anticancer drugs that are inhibitors of anaplastic lymphoma kinase (ALK), epidermal growth factor receptor (EGFR or HER1), human epidermal growth factor receptor 2 (HER2), human epidermal growth factor receptor 4 (HER4), fibroblast growth factor receptors (FGFRs), vascular endothelial growth factor receptors (VEGFRs), mesenchymal-epithelial transition factor (MET) or receptor tyrosine kinase rearranged during transfection (RET) (Table 1). These drugs show anticancer activity by blocking multiple molecular signal transduction pathways (Figure 1).
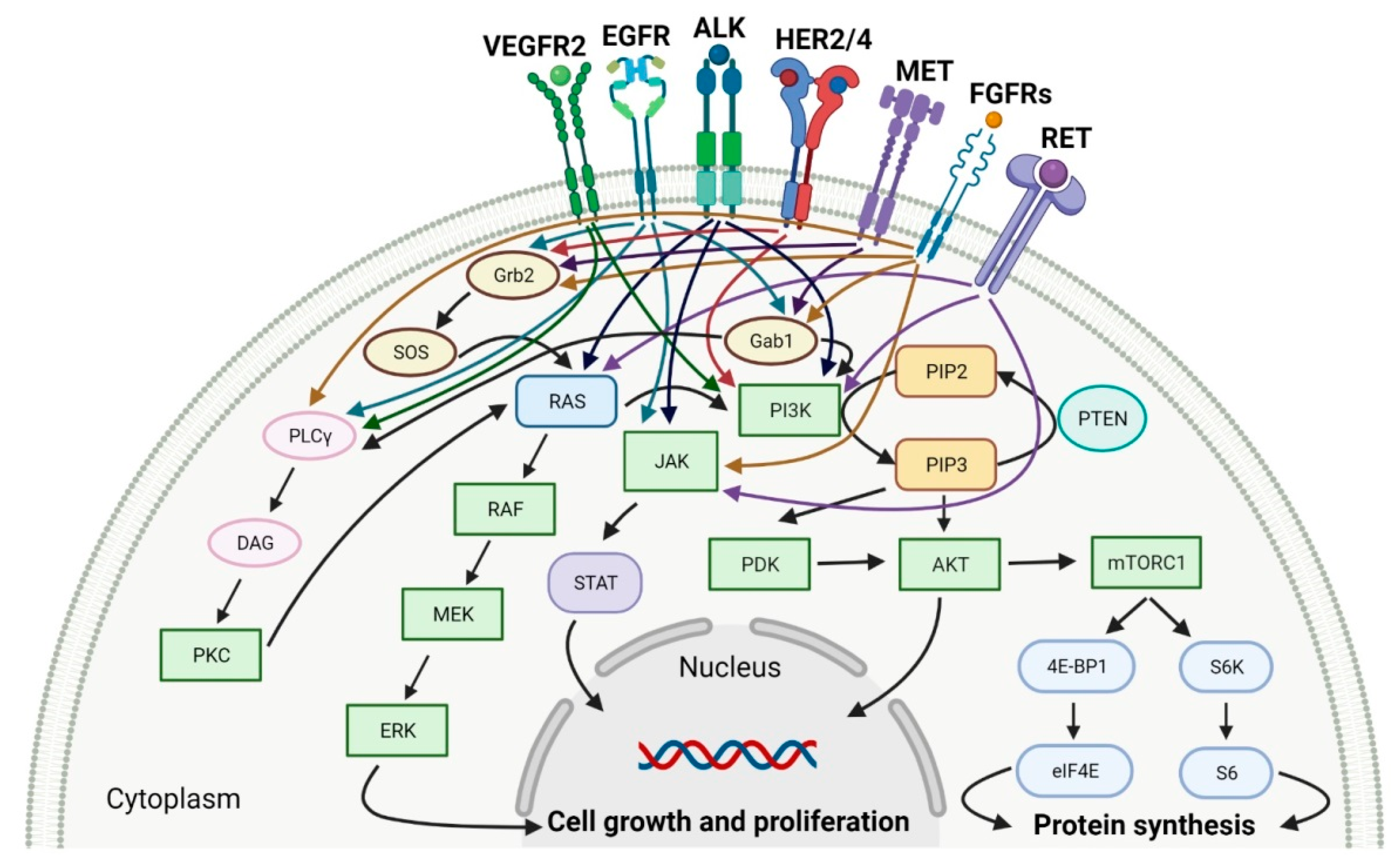
Figure 1. Molecular signal transduction pathways for specific receptor tyrosine kinases (RTKs). VEGFR2: vascular endothelial growth factor receptor 2. EGFR: epidermal growth factor receptor. ALK: anaplastic lymphoma kinase. HER2/4: human epidermal growth factor receptor 2 and 4. MET: mesenchymal-epithelial transition factor. FGFRs: fibroblast growth factor receptors. RET: tyrosine kinase rearranged during transfection receptor. Gab1: Grb2-associated-binding protein 1. Grb2: growth factor receptor-bound protein 2. SOS: Son of sevenless. PLCγ:. phospholipase C gamma. DAG: diacylglycerol. PKC: protein kinase C. RAS: rat sarcoma viral oncogene homolog. RAF: proto-oncogene serine/threonine-protein kinase. MEK: mitogen-activated protein kinase kinase. ERK: mitogen-activated protein kinase. PI3K: phosphatidylinositol 3-kinase. PIP2: phosphatidylinositol 4,5-bisphosphate. PIP3: phosphatidylinositol-3,4,5-trisphosphate. PTEN: phosphatase and tensin homolog deleted on chromosome ten. PDK: 3-phosphoinositide-dependent protein kinase. AKT: protein kinase B. mTORC1: mammalian target of rapamycin complex 1. 4E-BP1: 4E-binding protein 1. eIF4E: eukaryotic translation initiation factor 4E. S6K: p70S6 kinase. S6: S6 protein. JAK: Janus kinase. STAT: signal transducer and activator of transcription. Created with BioRender.com based on information in [3][4][5][6][7][8][9].
The oncogenic driver mutations identified in non-small-cell lung cancer (NSCLC) include ALK gene rearrangements, ROS1 gene rearrangements, EGFR mutations, MET mutations and RET rearrangements [10]. In NSCLC harboring ALK gene rearrangements are observed ALK fusion proteins with potent transforming activity as oncogenic drivers of tumor growth [11]. Ceritinib is the second-generation AKL inhibitor that blocks autophosphorylation of ALK and ALK-mediated phosphorylation of signal transducer and activator of transcription 3 (STAT3), which is a downstream signaling protein [12][13]. Hence, this drug inhibits the cell cycle in the G1 phase and the proliferation of ALK-dependent cancer cells. Among the existing therapies targeting EGFR-mutated NSCLC, there have been two FDA-approved medicaments during the last eleven years, i.e., osimertinib and mobocertinib. Osimertinib is a third-generation, irreversible TK inhibitor of both EGFR TKI-sensitizing mutations and a secondary EGFR mutation in exon 20, namely T790M [14]. Mobocertinib, on the other hand, is a first-in-class irreversible EGFR TK inhibitor, which was specifically developed to selectively inhibit oncogenic variants containing EGFR exon 20 insertion (EGFRex20ins) mutations. Both drugs form a covalent bond with cysteine 797 in EGFR with high-affinity binding resulting in sustained EGFR activity inhibition [15][16]. The difference in the structure of these drugs is the presence of an isopropyl ester group on the pyrimidine ring of mobocertinib, leading to increased selectivity for the EGFRex20ins mutant compared with osimertinib [16]. In NSCLC, MET and its mutant variants produced by gene mutation, amplification and overexpression are attractive targets for a blockade. For example, MET and variant with exon 14 skipping mutation are targets for capmatinib and tepotinib activity. The drugs act by inhibition of MET phosphorylation and the activation of key downstream effectors in MET-dependent cancer cell lines [17][18]. The cancers harboring RET alterations, particularly NSCLC, can be treated with pralsetinib. It selectively inhibits RET autophosphorylation and proliferation of RET-mutant cancer cells [9].
Overexpression of HER2 occurs approximately in 15 to 20% of breast cancers. Neratinib and tucatinib are inhibitors of the human epidermal growth factor receptors (HERs) that are used for the treatment of HER2-positive breast cancer (HER2 + BC). Neratinib irreversibly inhibits EGFR, HER2 and HER4 kinases, while tucatinib reversibly and highly selectively blocks HER2. The drugs have shown to be effective in monotherapy or in combination chemotherapy with capecitabine [19][20]. Patients with HER2 + BC who have disease progression after prior therapy with multiple HER2-targeted drugs may benefit from these TKIs used with or without trastuzumab [21][22]. The mechanism of action of both drugs includes binding to the ATP pocket of the HER2, which results in decreased receptor autophosphorylation and inhibition of downstream mitogen-activated protein kinase (MAPK) and phosphatidylinositol triphosphate kinase (PI3K) signaling. This leads to cell cycle arrest at the G1-S phase, thereby reducing cell proliferation [23][24].
FGFR2 fusion or rearrangements are present in 10–16% of intrahepatic cholangiocarcinomas. Treatment options, which improve clinical outcomes of patients with cholangiocarcinoma (CCA) harboring FGFR2 gene fusions, have been extended to the first two targeted therapies, i.e., pemigatinib and infigratinib [25][26]. The FDA approval of these TKIs includes the indication for adults with previously treated, unresectable, locally advanced or metastatic CCA. Their mechanism of action is a selective, ATP-competitive inhibition of fibroblast growth factor receptors (FGFRs). Both drugs potently inhibit FGFR1, FGFR2 and FGFR3 kinases and also demonstrate weaker activity against FGFR4 [27][28].
Renal cell carcinoma (RCC) is the most common type of kidney cancer. From a pathologist’s point of view, RCC tends to be a highly vascular tumor. The prominent vascularization is due to the increased production of proangiogenic growth factors, such as vascular endothelial growth factor receptors (VEGFRs) [29]. Tivozanib is a quinoline-urea derivative that inhibits VEGFRs in an ATP-competitive manner. In particular, the drug shows inhibitory activity against VEGFR-1, VEGFR-2 and VEGFR-3 at picomolar concentrations. The analysis of the mechanism of action indicates that tivozanib produced a significant inhibition of the ligand-induced phosphorylation of VEGFRs causing direct anticancer activity as well as suppression of angiogenesis and vascular permeability [30]. In clinical trials, this agent used as third-line or fourth-line therapy in patients with RCC improved progression-free survival and was better tolerated than sorafenib [31]. The promising results of tivozanib led to its approval by the FDA for the treatment of adult patients with relapsed or refractory advanced RCC following two or more prior systemic therapies [32].
Table 1. Features of the tyrosine kinase inhibitors approved as drugs by the Food and Drug Administration (FDA) from 2011 to 2022. The order of drugs is tabulated in order of most recent to oldest registration date. A generic name of a drug is an international nonproprietary name (INN).
| No | Generic Name of Drug | Brand Name and Company |
First FDA/EMA Approval Date | Structure | Molecular Target | Route of Administration | Indication | Adverse Effects | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Mobocertinib | EXKIVITY Takeda Pharmaceuticals America, Inc., Deerfield, IL, USA |
FDA: 15 September 2021 EMA: Not approved |
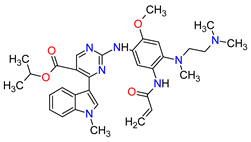 |
EGFR 1 | Oral | Non-Small Cell Lung Cancer | Diarrhea, rash, stomatitis, vomiting, decreased appetite, nausea, paronychia, musculoskeletal pain, dry skin, fatigue, decreased hemoglobin, decreased lymphocytes, increased creatinine, amylase, and lipase, decreased potassium, and magnesium | [33] |
| 2 | Infigratinib | TRUSELTIQ BridgeBio Pharma, Inc., Palo Alto, CA, USA | FDA: 28 May 2021 EMA: 21 August 2020 |
 |
FGFRs 2 | Oral | Cholangiocarcinoma | Nail toxicity, stomatitis, dry eye, fatigue, increased creatinine, phosphate, alkaline phosphate, and alanine aminotransferase, decreased phosphate, and hemoglobin | [34][35] |
| 3 | Tivozanib | FOTIVDA AVEO Oncology, Boston, MA, USA; Eusa Pharma (Netherlands) B.V., Schiphol-Rijk |
FDA: 10 March 2021 EMA: 24 August 2017 |
 |
VEGFRs 3 | Oral | Renal Cell Carcinoma | Fatigue, hypertension, diarrhea, decreased appetite, nausea, dysphonia, hypothyroidism, cough, stomatitis, sodium decreased, lipase increased, and phosphate decreased | |
| 4 | Tepotinib | TEPMETKO EMD Serono, Inc., Darmstadt, Germany. | FDA: 3 February 2021 EMA: Not approved |
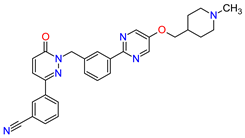 |
MET 4 | Oral | Non-Small Cell Lung Cancer | Peripheral edema, diarrhea, fatigue, nausea, decreased appetite, increased blood creatinine levels, hypoalbuminemia, increased amylase levels | [38] |
| 5 | Pralsetinib | GAVRETO Genentech, Inc., South San Francisco, CA, USA | FDA: 4 September 2020 EMA: 18 November 2021 |
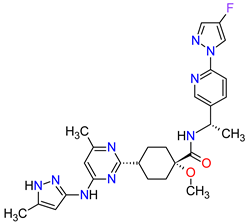 |
RET 5 | Oral | Non-Small Cell Lung Cancer | Fatigue, constipation, musculoskeletal pain, hypertension | [39][40] |
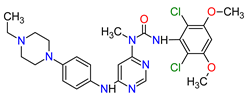
| 6 | Capmatinib | TABRECTA Novartis Pharmaceuticals Corporation, Basel, Switzerland | FDA: 6 May 2020 EMA: Not approved |
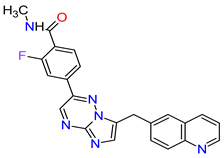 |
MET 4 | Oral | Non-Small Cell Lung Cancer | Peripheral edema, nausea, fatigue, vomiting, dyspnea, decreased appetite | [41] |
| 7 | Pemigatinib | PEMAZYRE Incyte Corporation, Wilmington, DE, USA | FDA: 17 April 2020 EMA: March 26, 2021 |
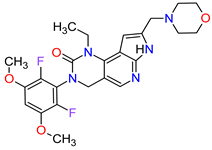 |
FGFRs 2 | Oral | Cholangiocarcinoma | Hyperphosphatasemia, alopecia, diarrhea, fatigue, dyspepsia | [42][43] |
| 8 | Tucatinib | TUKYSA Seattle Genetics, Inc., Bothell, WA, USA | FDA: 17 April 2020 EMA: 11 February 2021 |
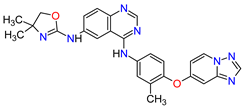 |
HER2 6 | Oral | Breast Cancer | Diarrhea, palmar–plantar erythrodysesthesia syndrome, decreased hemoglobin or phosphate, nausea | [44][45] |
| 9 | Neratinib | NERLYNX Puma Biotechnology, Inc., Los Angeles, CA, USA |
FDA: 17 July 2017 EMA: 31 August 2018 |
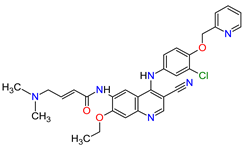 |
EGFR 1, HER2 6, HER4 7 | Oral | Breast Cancer | Diarrhea | [46][47] |
| 10 | Osimertinib | TAGRISSO AstraZeneca, Cambridge, UK | FDA: 13 November 2015 EMA: 24 April 2017 |
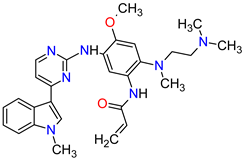 |
EGFR 1 | Oral | Non-Small Cell Lung Cancer | Diarrhea, rash, dry skin, nail toxicity | [48][49] |
| 11 | Ceritinib | ZYKADIA Novartis Pharmaceuticals Corporation, Basel, Switzerland | FDA: 29 April 2014 EMA: 6 May 2015 |
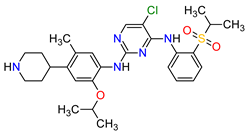 |
ALK 8 | Oral | Non-Small Cell Lung Cancer | Diarrhea, nausea, vomiting, abdominal pain | [50][51] |
1 EGFR: epidermal growth factor receptor. 2 FGFRs: fibroblast growth factor receptors. 3 VEGFRs: vascular endothelial growth factor receptors. 4 MET: mesenchymal-epithelial transition factor. 5 RET: tyrosine kinase rearranged during transfection receptor. 6 HER2: human epidermal growth factor receptor 2. 7 HER4: human epidermal growth factor receptor 4. 8 ALK: anaplastic lymphoma kinase.
2. Cyclin-Dependent Kinase (CDK) Inhibitors
Cyclin-dependent kinases (CDKs) are a family of serine-threonine kinases. They regulate the cell cycle and other important cellular functions, including gene transcription, metabolism and neuronal function. The human genome codifies 20 CDKs (1–20) and 13 groups of cyclin, which are proteins that control the activities of the CDKs through their oscillating level during the cell cycle [52]. The CDKs have been grouped into cell cycle-related subfamilies (CDK1, 4 and 5) and transcriptional subfamilies (CDK7, 8, 9, 11 and 20). Dysregulating the CDKs and cyclins level leads to abnormal cell proliferation and tumor growth. Owing to the role of CDKs in cancer cells, their inhibition is an important target for novel anticancer drugs. The suppression of CDK4 and CDK6 activity is now being investigated to treat various solid tumors, including lung, prostate and ovarian cancers. The CDK4/6 inhibitors, i.e., palbociclib, ribociclib and abemaciclib, demonstrated promising clinical activity in the treatment of advanced breast cancer, thereby being recently FDA approved (Table 2) [53][54]. The approval of abemaciclib (as VERZENIO) includes using it for monotherapy or in combination with fulvestrant, which is an estrogen receptor antagonist. Palbociclib (as IBRANCE) was registered for combination therapy with fulvestrant or an aromatase inhibitor (letrozole). Ribociclib (as KISQALI) was approved only in combination with an aromatase inhibitor (letrozole) for initial endocrine-based therapy. All of these drugs are selective inhibitors of cyclin-dependent kinase 4 (CDK4) and 6 (CDK6). They inhibit Rb protein phosphorylation in the early G1 phase, thereby blocking cell-cycle transition from G1 to S phase and reducing cancer cell growth (Figure 2) [55][56][57][58]. In addition, abemaciclib is able to penetrate the blood–brain barrier (BBB), thereby being promising to achieve regression of primary and metastatic tumors involving the central nervous system (CNS). The ongoing clinical trials allow evaluating abemaciclib for therapy in patients with brain metastases and leptomeningeal metastases (LM) secondary to HR-positive breast cancer [59][60].
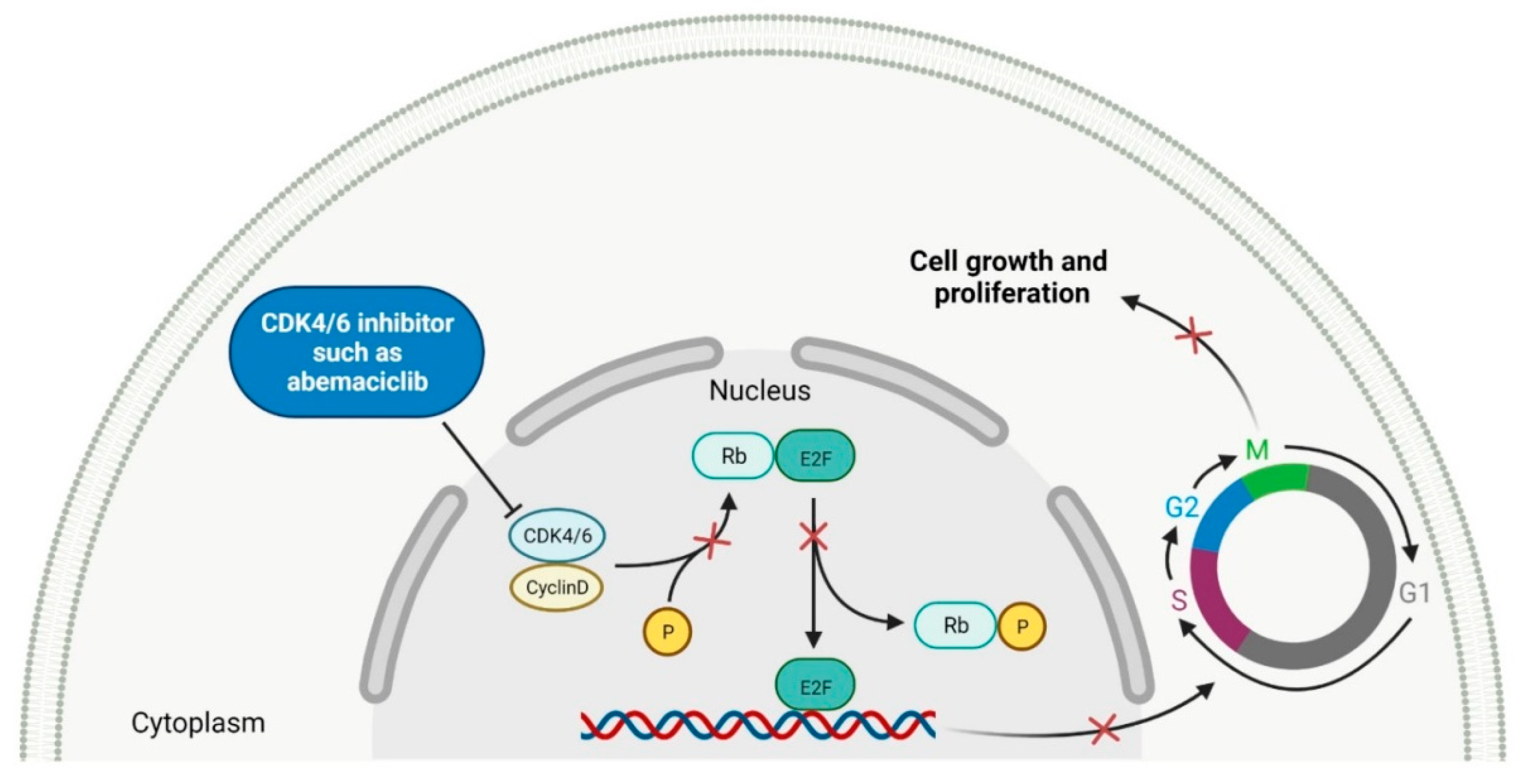
Figure 2. Mechanism of action of CDK4/6 inhibitors (the “x” on the arrows indicates process inhibition). CDK4/6: cyclin-dependent kinase 4/6. P: phosphate group. Rb: retinoblastoma protein. E2F: E2 factor. G1: first growth phase. S: synthesis phase. G2: second growth phase. M: mitotic phase. Created with BioRender.com based on information in Ref. [54].
Table 2. Features of the cyclin-dependent kinase inhibitors approved as drugs by the Food and Drug Administration (FDA) from 2011 to 2022. The order of drugs is tabulated in order of most recent to oldest registration date. A generic name of a drug is an international nonproprietary name (INN).
| No. | Generic Name of Drug | Brand Name and Company |
First FDA/EMA Approval Date | Structure | Molecular Target | Route of Administration | Indication | Adverse Effects | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Abemaciclib | VERZENIO Eli Lilly and Company, Indianapolis, IN, USA |
FDA: 28 September 2017 EMA: 27 September 2018 |
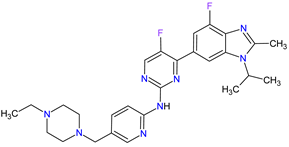 |
CDK4 1, CDK6 2 | Oral | Breast Cancer | Diarrhea, fatigue, nausea, decreased appetite, abdominal pain, neutropenia, vomiting, infections, anemia, headache, thrombocytopenia, leucopenia | [61][62] |
| 2 | Ribociclib | KISQALI Novartis Pharmaceuticals Corporation, Basel, Switzerland | FDA: 13 March 2017 EMA: 22 August 2017 |
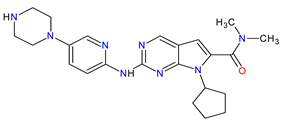 |
CDK4 1, CDK6 2 | Oral | Breast Cancer | Neutropenia, nausea, infections, fatigue, diarrhea | [63][64] |
| 3 | Palbociclib | IBRANCE Pfizer Inc., New York City, NY, USA |
FDA: 3 February 2015 EMA: 9 November 2016 |
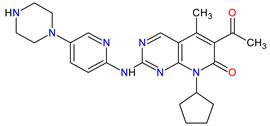 |
CDK4 1, CDK6 2 | Oral | Breast Cancer | Neutropenia, leukopenia, fatigue, anemia, nausea, arthralgia, alopecia, diarrhea, hot flush | [65][66] |
1 CDK4: cyclin-dependent kinase 4. 2 CDK6: cyclin-dependent kinase 6.
3. Multi-Kinase Inhibitors
In solid cancers, the frequently aberrant activity of various components of signaling pathways occurs by the hyperactivation of several different kinases (Figure 3). Multi-kinase inhibitor is one agent that targets a set of structurally related kinases leading to simultaneous blocking of their activity [67]. The use of one multi-kinase inhibitor is preferred to two single agents, since drug–drug interactions can trigger changing metabolism and activities against particular kinases. Multi kinase drugs become the second choice when their pharmacokinetic properties are worse. Besides, multi-kinase inhibitors are less specific and can consequently result in more side effects. The disadvantage during treatment with multi-kinase inhibitors is acquired resistance [68]. The approval characteristics of FDA-registered multi-kinase inhibitors are presented in Table 3.
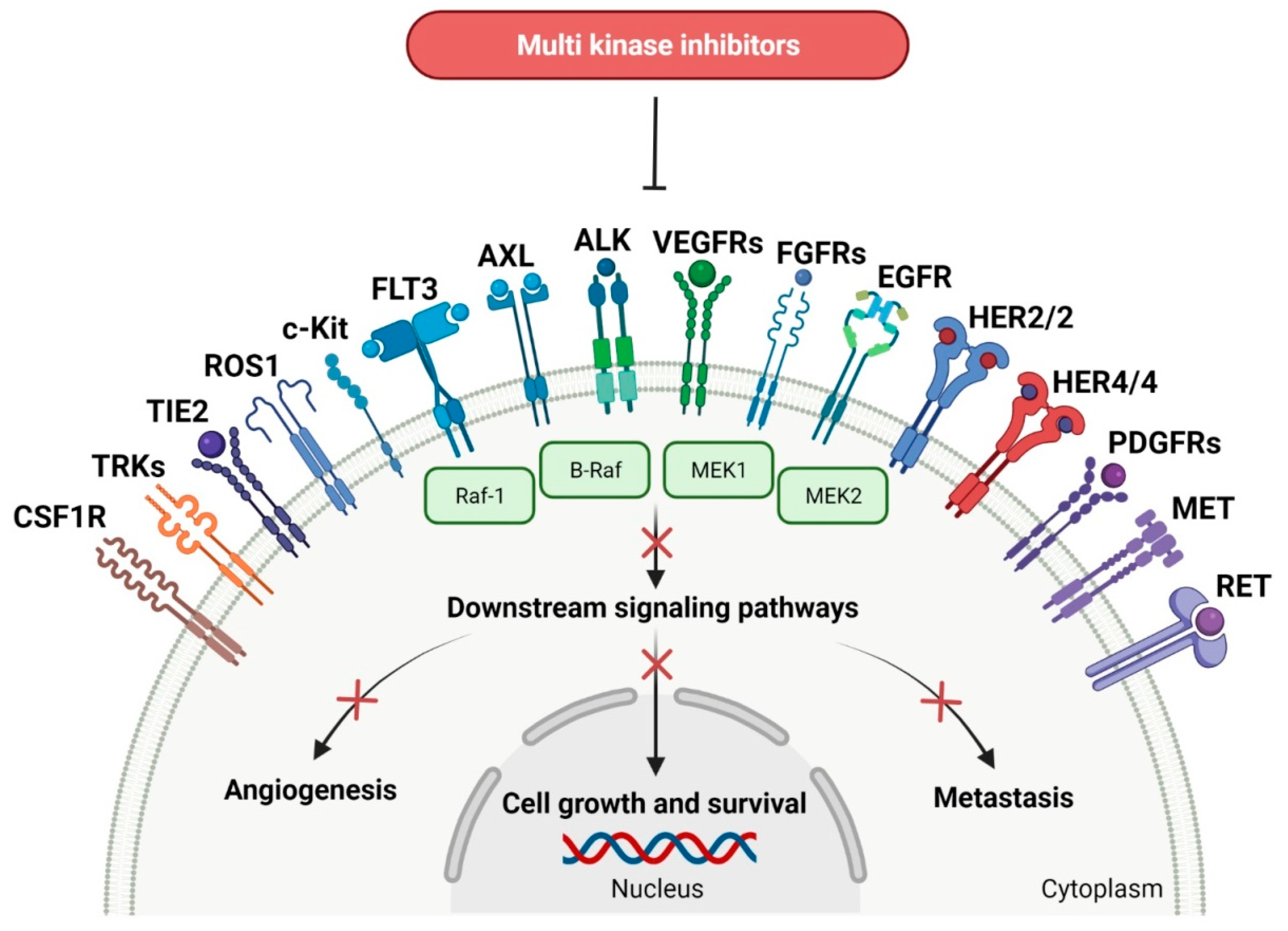
Figure 3. Schematic representation of mode of action of multi-kinase inhibitors that target a set of various related kinases (the “x” on the arrows indicates process inhibition). CSF1R: colony-stimulating factor 1 receptor. TRKs: tropomyosin receptor tyrosine kinases. TIE2: tunica interna endothelial cell kinase 2. ROS1: proto-oncogene tyrosine-protein kinase ROS. c-Kit: mast/stem cell growth factor receptor. FLT3: FMS-like tyrosine kinase-3. AXL: AXL receptor tyrosine kinase. ALK: anaplastic lymphoma kinase. VEGFRs: vascular endothelial growth factor receptors. FGFRs: fibroblast growth factor receptors. EGFR: epidermal growth factor receptor. HER2/2: human epidermal growth factor receptor 2 and 2. HER4/4: human epidermal growth factor receptor 4 and 4. PDGFRs: platelet-derived growth factor receptors. RET: receptor tyrosine kinase rearranged during transfection. B-Raf: serine/threonine-protein kinase B-Raf. Raf-1: RAF serine/threonine-protein kinase. MEK1: mitogen-activated protein kinase kinase 1. MEK2: mitogen-activated protein kinase kinase 2. MET: mesenchymal-epithelial transition factor. Created with BioRender.com.
Patients with NSCLC receiving the first-generation TKIs, e.g., crizotinib, geftinib and erlotinib, experienced issues related to acquired resistance. This resistance can develop by various mechanisms, such as crizotinib-resistant mutations in the anaplastic lymphoma kinase (ALK) domain. In addition, patients’ treatment with crizotinib often develops CNS metastases, likely due to the poor CNS penetration of crizotinib. However, crizotinib exhibits higher clinical response rates than standard chemotherapy and is recommended both for first-line therapy in NSCLC, as well as next-line therapy in patients who have not been treated with crizotinib previously. The next-generation multi-kinase inhibitors are designed to overcome TKI-resistant mutations. Alectinib, brigatinib and entrectinib, which are the second-generation ALK inhibitors, possess activity against treatment-resistant ALK mutants, whereas lorlatinib, which belongs to the third-generation drug, is highly selective proto-oncogene tyrosine-protein kinase ROS (ROS1) and ALK inhibitor and has the ability of robust brain penetration [69]. The second-generation EGFR TKIs, namely dacomitinib and afatinib, are characterized by their broader activity against HER family members and irreversibility, covalently binding to their targets of the kinases domain. They have the potential for anticancer activity against receptors with acquired mutations that are resistant to first-generation inhibitors. For example, dacomitinib specifically inhibits EGFR with exon 19 deletion or exon 21 L858R substitution mutations but also inhibits HER2, HER4 and transphosphorylation of HER3 [70].
Fibroblast growth factor receptor (FGFR) mutations are frequently observed in a variety of malignancies, e.g., FGFR2/3 alternations are common in urothelial carcinoma. Erdafitinib, a pan-FGFR inhibitor, is a promising therapy for cancers harboring these mutations. Erdafitinib obtained its first global approval in 2019 for the treatment of adult patients with locally advanced or metastatic urothelial carcinoma with FGFR alterations. The response to treatment was fast and independent of the number of previous therapies, the presence of visceral metastasis or tumor location [71]. The ongoing clinical trials show that erdafitinib demonstrated anticancer activity against other cancers, including cholangiocarcinoma, liver cancer, non-small cell lung cancer, prostate cancer, lymphoma and esophageal cancer [72]. The next drug approved by the FDA in 2019 is pexidartinib, which is used in the therapy of symptomatic tenosynovial giant cell tumor (TGCT). The drug is a selective inhibitor of the colony-stimulating factor 1 (CSF1) receptor, mast/stem cell growth factor receptor (c-Kit or CD117) and FMS-like tyrosine kinase 3 harboring an internal tandem duplication mutation (FLT3-ITD). The action mechanism of pexidartinib is to arrest the kinase in the autoinhibited state by interacting with the CSF1R juxtamembrane region, which prevents an ATP and substrate binding [73].
The multi-kinase inhibitors that already obtained approval for the treatment of metastatic melanoma, an aggressive form of skin cancer with a high mortality rate, are second-generation serine/threonine-protein kinase B-Raf (B-Raf) inhibitors, such as vemurafenib, dabrafenib or encorafenib and mitogen-activated protein kinase (MAPK) kinase (MEK) inhibitors, such as trametinib, cobimetinib or binimetinib. They are the most promising treatment strategies for melanoma consisting of selective inhibition of the active conformation of the B-Raf, especially with V600E mutation [74]. Furthermore, dabrafenib and encorafenib are also used in the therapy of cancers with several other mutated forms of B-Raf, e.g., V600K-mutated melanomas and V600K/D-mutated melanoma, respectively. The B-Raf inhibitors are characterized by high response rates, a mild, manageable toxicity profile and improved progression-free survival (PFS) as compared with chemotherapy, but their use is limited by the rapid development of resistance [75]. Encorafenib is distinguished among the other second-generation B-Raf kinase inhibitors by increasing its inhibitory effect with a shorter off-rate [76]. Currently, monotherapy with B-Raf inhibitor for the treatment of BRAF-mutated melanoma is subsequently replaced by combination therapy with B-Raf and MEK inhibitors, which target key enzymes in the MAPK signaling pathway (RAS-RAF-MEK-ERK). The approved combination of active anticancer ingredients, such as vemurafenib plus cobimetinib, dabrafenib plus trametinib or encorafenib plus binimetinib, is a more effective therapy than B-Raf inhibitor monotherapy and is recommended as a first-line therapeutic option in treating melanoma [77]. What is more, encorafenib and binimetinib combination therapy is already ongoing clinical development for the treatment of colorectal cancer (CRC). Selumetinib, the next inhibitor of MEK 1 and 2, is approved in children with neurofibromatosis type 1 and inoperable plexiform neurofibromas. The long-term treatment with selumetinib has meaningful benefits, such as a high level of clinical response and absence of cumulative toxic effects [78].
In 2020, the combination of encorafenib and monoclonal antibody (cetuximab) received its first approval for treating metastatic colorectal cancer (mCRC) [79]. CRC can also be treated with regorafenib, which is an inhibitor of VEGFR-1, VEGFR-2 and VEGFR-3, tunica interna endothelial cell kinase 2 (TIE2), PDGFRB, c-Kit, FGFR1, RET, RAF proto-oncogene serine/threonine-protein kinase (RAF-1) and B-Raf, including wild-type B-Raf and B-Raf V600E [80]. The inhibitor possesses antiangiogenic activity due to the inhibition of TIE2. Moreover, it has anticancer activity against gastrointestinal stromal tumor (GIST), hepatocellular carcinoma and is ongoing clinical development for various malignant tumors. Another two drugs, which were approved in 2020 for GIST, are avapritinib and ripretinib. They inhibit mast/stem cell growth factor receptor (c-Kit) and platelet-derived growth factor receptor α (PDGFRA). Avapritinib is a therapy only for GIST harboring a PDGFRA exon 18 mutations, including PDGFRA D842V mutations, whereas ripretinib inhibits wild-type c-Kit and PDGFRA mutations, as well as multiple primary and secondary resistance mutations in GIST [81]. Ripretinib is an appropriate treatment for patients who were resistant to other approved tyrosine kinase inhibitors, such as regorafenib or imatinib. The mechanism of its action involves durably binding to both the switch pocket in the intracellular juxtamembrane domain and the activation loop in the kinase domain to prevent from adopting an active state of kinase and locking it in the inactive conformation, thereby inhibiting cell proliferation [82].
A total of six drugs, which have been approved by the FDA since 2011 for thyroid cancer treatment, are antiangiogenic multi-kinase inhibitors, including vandetanib, cabozantinib and lenvatinib or mutation-specific inhibitors, including dabrafenib for BRAF-mutated anaplastic thyroid cancer (ATC), larotrectinib for NTRK-fusion thyroid cancer and selpercatinib for RET-mutant medullary thyroid cancer (MTC). Vandetanib and cabozantinib are registered for the treatment of advanced MTC. The drugs inhibit EGF, RET and VEGF receptors or the MET, RET and VEGF receptors, respectively. Thus, their action involves blocking the sustaining proliferative signaling mediated by tyrosine kinase receptors, angiogenesis and apoptosis. Cabozantinib, due to downregulation of the MET pathway, may prevent invasiveness and metastatic spread of cancer cells and the development of acquired resistance. Hence, it induces more prolonged clinical responses than those to other TKIs. What is more, it displays stronger antiangiogenic activity than vandetanib [83]. However, the use of cabozantinib and vandetanib is at least partially limited by their adverse events. In contrast, next-generation drug selpercatinib, which is a highly potent and selective RET inhibitor, shows durable efficacy with a more satisfactory safety profile [84]. The first FDA-approved treatment for patients with anaplastic thyroid carcinomas (ATCs), a highly aggressive and undifferentiated cancer, is dabrafenib (B-Raf inhibitor) plus trametinib (MEK inhibitor). This dual inhibition improves overall response frequency and achieves better clinical results compared with B-Raf inhibitor monotherapy [85].
Larotrectinib is a highly selective TRK inhibitor that was developed for the therapy for cancers with a neurotrophic receptor tyrosine kinase (NTRK) gene fusion in adults and children [86]. All patients undergoing the larotrectinib treatment were characterized by advanced solid tumors, including salivary gland tumors, infantile fibrosarcoma, thyroid cancer, NSCLC and other cancers. Larotrectinib also has potential efficacy against CNS tumors because of its ability to cross the blood–brain barrier [87]. The only other registered TRK inhibitor apart from larotrectinib is entrectinib, with activity against ALK, TRK and ROS1. In clinical trials, responses to entrectinib treatment were observed in the following diseases: NSCLC, mammary analog secretory carcinoma (MASC), colorectal cancer, melanoma, glioneuronal tumor and renal cell carcinoma (RCC) [88]. Lenvatinib is also used in therapy for RCC, as well as radioiodine-refractory differentiated thyroid cancer (RR-DTC), hepatocellular carcinoma and endometrial cancer. The inhibitor targets VEGFR-1, VEGFR-2, VEGFR-3, FGFR1-3, RET, mast/stem cell growth factor receptor (c-Kit) and platelet-derived growth factor receptor β (PDGFRB), thereby resulting in broad spectrum of direct antitumor activity and significant antiangiogenic effects [89]. Another drug for RCC is axitinib, which is approved for monotherapy in the second-line treatment and in combination with pembrolizumab or avelumab for first-line therapy [90][91]. It inhibits both proliferation and angiogenesis through blocking receptors, such as c-Kit and PDGFR on the one hand, and proangiogenic receptors VEGFR-1, VEGFR-2 and VEGFR-3 on the other. Axitinib has demonstrated promising activity in other solid tumors as well, including metastatic breast cancer, advanced NSCLC, pancreatic and thyroid cancers [92].
Table 3. Features of the multi-kinase inhibitors approved as drugs by the Food and Drug Administration (FDA) from 2011 to 2022. The order of drugs is tabulated in order of most recent to oldest registration date. A generic name of a drug is an international nonproprietary name (INN).
| No. | Generic Name of Drug | Brand Name and Company |
First FDA/EMA Approval Date | Structure | Molecular Target | Route of Administration | Indication | Adverse Effects | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Ripretinib | QINLOCK Deciphera Pharmaceuticals, Inc., Waltham, MA, USA | FDA: 15 May 2020 EMA: 18 November 2021 |
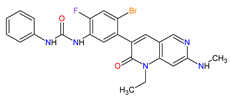 |
c-Kit 1, PDGFRA 2 | Oral | Gastrointestinal Stromal Tumor | Alopecia, fatigue, nausea, abdominal pain, constipation, myalgia, diarrhea, decreased appetite, palmar–plantar erythrodysesthesia syndrome, vomiting | [93][94] |
| 2 | Selpercatinib | RETEVMO Eli Lilly and Company, Indianapolis, IN, USA |
FDA: 8 May 2020 EMA: 11 February 2021 |
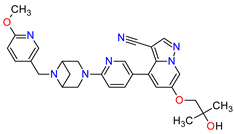 |
RET 3 | Oral | Non-Small Cell Lung Cancer, Thyroid Cancer | Increased AST levels, increased glucose levels, decreased albumin levels, decreased leukocyte levels, decreased calcium levels, increased creatinine levels, dry mouth, diarrhea, increased alkaline phosphatase levels, hypertension, fatigue, decreased platelet levels, edema, increased total cholesterol levels, decreased sodium levels, rash, constipation, decreased magnesium levels, increased potassium levels, increased bilirubin levels, headache, decreased glucose levels, nausea, abdominal pain, cough, prolonged QT interval, dyspnea, vomiting, hemorrhage | [95][96] |
| 3 | Selumetinib | KOSELUGO AstraZeneca, Cambridge, UK | FDA: 13 April 2020 EMA: 17 June 2021 |
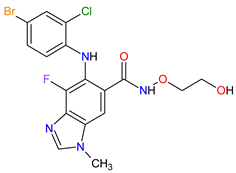 |
MEK1 4, MEK2 5 | Oral | Neurofibromatosis Type 1 | Vomiting, rash, abdominal pain, diarrhea, nausea, dry skin, musculoskeletal pain, fatigue, pyrexia, stomatitis, acneiform rash, headache, paronychia, pruritus, dermatitis, constipation, hair changes, epistaxis, hematuria, proteinuria, decreased appetite, decreased cardiac ejection fraction, edema, sinus tachycardia, skin infection | [97][98] |
| 4 | Avapritinib | AYVAKIT Blueprint Medicines Corporation, Cambridge, MA, USA | FDA: 9 January 2020 EMA: 24 September 2020 |
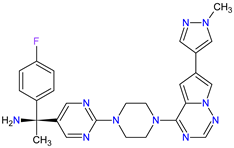 |
c-Kit 1, PDGFRA 2 | Oral | Gastrointestinal Stromal Tumor | Edema, nausea, fatigue/asthenia, cognitive impairment, vomiting, decreased appetite, diarrhea, increased lacrimation, abdominal pain | [99][100] |
| 5 | Entrectinib | ROZLYTREK Genentech, Inc., South San Francisco, CA, USA | FDA: 15 August 2019 EMA: 31 July 2020 |
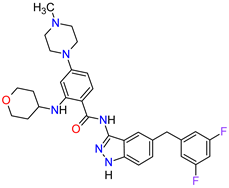 |
TRK 6, ROS1 7, ALK 8 | Oral | Solid Tumors, Non-Small Cell Lung Cancer | Dysgeusia, fatigue, dizziness, constipation, nausea, diarrhea, increased weight, paresthesia, increased blood creatinine, myalgia, peripheral edema, vomiting, anemia, arthralgia, increased aspartate aminotransferase (AST) | [101][102] |
| 6 | Pexidartinib | TURALIO Daiichi Sankyo, Tokyo, Japan |
FDA: 2 August 2019 EMA: Not approved |
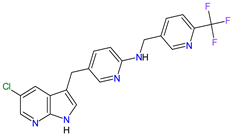 |
CSF1R 9, c-Kit 1, FLT3 10 | Oral | Tenosynovial Giant Cell Tumor | Hair color changes (depigmentation), fatigue, increased AST, increased alanine aminotransferase (ALT), dysgeusia, vomiting, periorbital edema, abdominal pain, decreased appetite, pruritus, hypertension, increased alkaline phosphatase | [103][104] |
| 7 | Erdafitinib | BALVERSA Janssen Pharmaceuticals, Inc., Raritan (HQ), NJ, USA | FDA: 12 April 2019 EMA: Not approved |
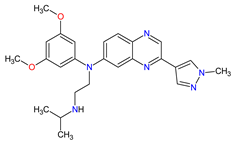 |
FGFRs 11 (1, 2, 3, 4) | Oral | Urothelial Carcinoma | Increased phosphate levels, stomatitis, fatigue, diarrhea, dry mouth, onycholysis, decreased appetite, dysgeusia, dry skin, dry eye, alopecia, palmar–plantar erythrodysaesthesia syndrome, constipation, abdominal pain, nausea, musculoskeletal pain |
[72] |
| 8 | Larotrectinib | VITRAKVI Loxo Oncology, Inc., Stamford, CT, USA |
FDA: 26 November 2018 EMA: 19 September 2019 |
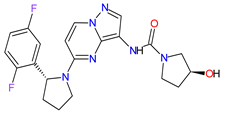 |
TRK 6 | Oral | TRK Fusion Cancers | Fatigue, nausea, dizziness, vomiting, anemia, increased transaminase levels, cough, constipation, diarrhea | [105][106] |
| 9 | Lorlatinib | LORBRENA Pfizer Inc., New York City, NY, USA | FDA: 2 November 2018 EMA: 6 May 2019 |
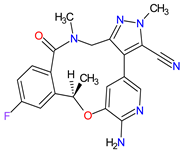 |
ALK 8, ROS1 7 | Oral | Non-Small Cell Lung Cancer | Hypercholesterolemia, hypertriglyceridemia, edema, peripheral neuropathy | [107][108] |
| 10 | Dacomitinib | VIZIMPRO Pfizer Inc., New York City, NY, USA |
FDA: 27 September 2018 EMA: 2 April 2019 |
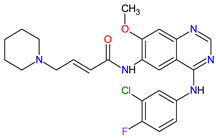 |
EGFR 12, HER2 13, HER4 14 | Oral | Non-Small Cell Lung Cancer | Diarrhea, paronychia, dermatitis acneiform, stomatitis, decreased appetite | [109][110] |
| 11 | Encorafenib | BRAFTOVI Pfizer Inc., New York City, NY, USA |
FDA: 27 June 2018 EMA: 20 September 2018 |
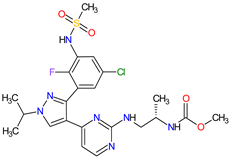 |
B-Raf 15 | Oral | Melanoma Metastatic, Colorectal Cancer | Nausea, diarrhea, vomiting, fatigue, arthralgia | [111][112] |
| 12 | Binimetinib | MEKTOVI Array BioPharma Inc., Boulder, CO, USA |
FDA: 27 June 2018 EMA: 20 September 2018 |
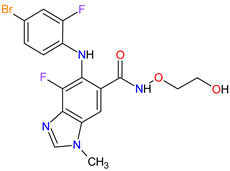 |
MEK1 4, MEK2 5 | Oral | Melanoma Metastatic | Nausea, diarrhea, vomiting, fatigue, arthralgia |
[111][113] |
| 13 | Brigatinib | ALUNBRIG Takeda Pharmaceuticals America, Inc., Deerfield, IL, USA |
FDA: 28 April 2017 EMA: 22 November 2018 |
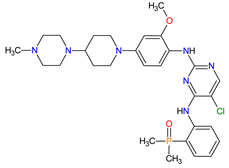 |
ALK 8, EGFR 12 | Oral | Non-Small Cell Lung Cancer | Nausea, diarrhea, fatigue, cough, headache, CPK elevation, pancreatic enzyme elevation, hyperglycemia | [114][115] |
| 14 | Alectinib | ALECENSA Genentech, Inc., South San Francisco, CA, USA | FDA: 11 December 2015 EMA: 16 February 2017 |
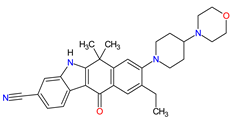 |
ALK 8 | Oral | Non-Small Cell Lung Cancer | Constipation, nausea, diarrhea, vomiting, edema, increased levels of bilirubin, AST and ALT, myalgia, rash, anemia, increase in bodyweight | [116][117] |
| 15 | Cobimetinib | COTELLIC Genentech, Inc., South San Francisco, CA, USA | FDA: 10 November 2015: EMA: 20 November 2015. |
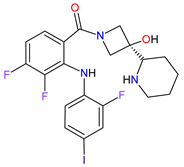 |
MEK1 4, MEK2 5 | Oral | Melanoma Metastatic | Diarrhea, nausea, rash, arthralgia, fatigue, increased creatine phosphokinase levels | [118][119] |
| 16 | Lenvatinib | LENVIMA Eisai Inc., Tokyo, Japan, U.S. Corporate Headquarters in Nutley, NJ, USA |
FDA: 13 February 2015 EMA: 28 May 2015 |
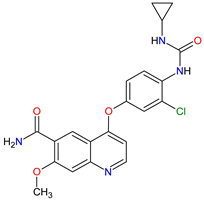 |
VEGFRs 16 (1, 2, 3), FGFR 11 (1, 2, 3, 4), PDGFRA 2, RET 3, c-Kit 1 | Oral | Thyroid Cancer, Renal Cell Carcinoma, Hepatocellular Carcinoma, Endometrial Cancer | Hypertension, diarrhea, fatigue or asthenia, decreased appetite, bodyweight decreased, nausea, stomatitis, palmar–plantar erythrodysethaesia syndrome, proteinuria | [120][121] |
| 17 | Afatinib | GILOTRIF Boehringer Ingelheim Pharmaceuticals, Inc., Ingelheim, Germany | FDA: 12 July 2013 EMA: 25 September 2013 |
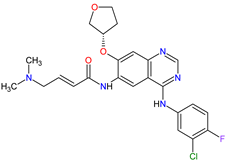 |
EGFR 12, HER2 13, HER4 14 | Oral | Non-Small Cell Lung Cancer | Diarrhea, rash/acne, stomatitis/mucositis, paronychia, dry skin, decreased appetite, pruritus, nausea, fatigue, vomiting, epistaxis, cheilitis | [122][123] |
| 18 | Trametinib | MEKINIST GlaxoSmithKline, London, UK | FDA: 29 May 2013 EMA: 30 June 2014 |
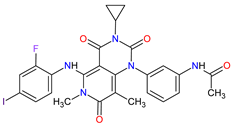 |
MEK1 4, MEK2 5 | Oral | Melanoma, Metastatic, Non-Small Cell Lung Cancer, Thyroid Cancer | Rash, diarrhea, fatigue, nausea/vomiting, peripheral edema | [124][125] |
| 19 | Dabrafenib | TAFINLAR GlaxoSmithKline, London, UK | FDA: 29 May 2013 EMA: 26 August 2013 |
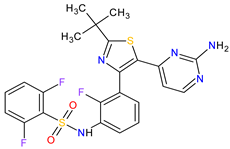 |
B-Raf 15 | Oral | Melanoma, Metastatic, Non-Small Cell Lung Cancer, Thyroid Cancer | Alopecia, arthralgia, back pain, constipation, cough, erythrodysaesthesia, fever, headache, hyperkeratosis, muscle pain, nasopharyngitis, papilloma, squamous cell cancer | [126][127] |
| 20 | Cabozantinib | CABOMETYX Exelixis, Inc., Alameda, CA, USA | FDA: 25 April 2016 EMA: 9 September 2016 |
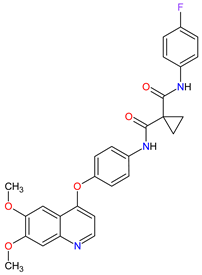 |
MET 17, RET 3, VEGFRs 16 (1, 2, 3), c-Kit 1, FLT-3 10, TIE2 18, TRKB 19, AXL 20 | Oral | Renal Cell Carcinoma, Hepatocellular Carcinoma | Diarrhea, fatigue, nausea, vomiting, decreased appetite, hypertension, palmar–plantar erythrodysesthesia syndrome | [128][129][130] |
| 21 | Cabozantinib | COMETRIQ Exelixis, Inc., Alameda, CA, USA |
FDA: 29 November 2012 EMA: 21 March 2014 |
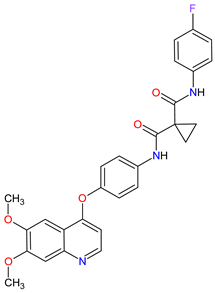 |
MET 17, RET 3, VEGFRs 16 (1, 2, 3), c-Kit 1, FLT-3 10, TIE2 18, TRKB 19, AXL 20 | Oral | Thyroid Cancer | Diarrhea, stomatitis, palmar–plantar erythrodysesthesia syndrome, decreased weight, decreased appetite, nausea, fatigue, oral pain, hair color changes, dysgeusia, hypertension, abdominal pain, constipation, increased AST, increased ALT, lymphopenia, increased alkaline phosphatase, hypocalcemia, neutropenia, thrombocytopenia, hypophosphatemia, and hyperbilirubinemia | [131][132][133] |
| 22 | Regorafenib | STIVARGA Bayer HealthCare Pharmaceuticals Inc., Whippany, NJ, USA |
FDA: 27 September 2012 EMA: 26 August 2013 |
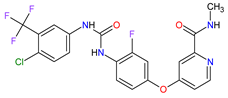 |
VEGFRs 16 (1, 2, 3), RET 3, c-Kit 1, PDGFRs 21 (A, B), FGFRs 11 (1, 2), TIE2 18, B-Raf 15, RAF-1 22 | Oral | Colorectal Cancer, Gastrointestinal Stromal Tumor, Hepatocellular Carcinoma | Asthenia/fatigue, decreased appetite and food intake, hand-foot skin reaction, palmar–plantar erythrodysesthesia, diarrhea, mucositis, weight loss, infection, hypertension, dysphonia | [134][135][136] |
| 23 | Axitinib | INLYTA Pfizer Inc., New York City, NY, USA |
FDA: 27 January 2012 EMA: 3 September 2012 |
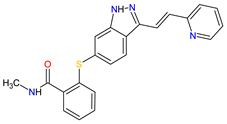 |
VEGFRs 16 (1, 2, 3), c-Kit 1, PDGFRs 21 (A, B) | Oral | Renal Cell Carcinoma | Diarrhea, hypertension, fatigue, decreased appetite, nausea, dysphonia, palmar–plantar erythrodysesthesia (hand-foot) syndrome, weight decreased, vomiting, asthenia, constipation | [137][138][139] |
| 24 | Crizotinib | XALKORI Pfizer Inc., New York City, NY, USA |
FDA: 26 August 2011 EMA: 23 October 2012 |
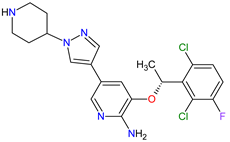 |
ALK 8, MET 17, ROS1 7 | Oral | Non-Small Cell Lung Cancer | Vision disorders, nausea, diarrhea, vomiting, edema, constipation, elevated transaminases, fatigue, decreased appetite, upper respiratory infection, dizziness, neuropathy | [140][141][142] |
| 25 | Vemurafenib | ZELBORAF Genentech, Inc., South San Francisco, CA, USA | FDA: 17 August 2011 EMA: 17 February 2012 |
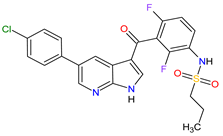 |
B-Raf 15 | Oral | Melanoma Metastatic | Arthralgia, rash, alopecia, fatigue, photosensitivity reaction, nausea, | [143][144][145] |
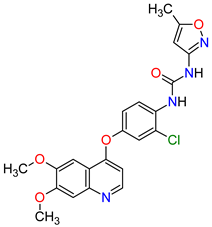
| 26 | Vandetanib | CAPRELSA AstraZeneca, Cambridge, UK | FDA: 6 April 2011 EMA: 17 February 2012 |
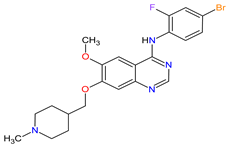 |
VEGFR-2 23, EGFR 12, RET 3 | Oral | Thyroid Cancer | Diarrhea, rash, nausea, hypertension, fatigue, headache, decreased appetite, acne, dermatitis acneiform, dry skin, photosensitivity reaction, erythema | [146][147] |
1 c-Kit: mast/stem cell growth factor receptor. 2 PDGFRA: platelet-derived growth factor receptor α. 3 RET: receptor tyrosine kinase rearranged during transfection. 4 MEK1: mitogen-activated protein kinase kinase 1. 5 MEK2: mitogen-activated protein kinase kinase 2. 6 TRK: tropomyosin receptor tyrosine kinase. 7 ROS1: proto-oncogene tyrosine-protein kinase ROS. 8 ALK: anaplastic lymphoma kinase. 9 CSF1R: colony-stimulating factor 1 receptor. 10 FLT3: FMS-like tyrosine kinase-3. 11 FGFRs: fibroblast growth factor receptors. 12EGFR: epidermal growth factor receptor. 13 HER2: human epidermal growth factor receptor 2. 14 HER4: human epidermal growth factor receptor 4. 15 B-Raf: serine/threonine-protein kinase B-Raf. 16 VEGFRs: vascular endothelial growth factor receptors. 17 MET: mesenchymal-epithelial transition factor. 18 TIE2: tunica interna endothelial cell kinase 2. 19 TRKB: tropomyosin receptor kinase B. 20 AXL: AXL receptor tyrosine kinase. 21 PDGFRs: platelet-derived growth factor receptors. 22 RAF-1: RAF proto-oncogene serine/threonine-protein kinase. 23 VEGFR-2: vascular endothelial growth factor receptor-2.
This entry is adapted from the peer-reviewed paper 10.3390/molecules27072259
References
- Manash Paul; Anup K. Mukhopadhyay; Tyrosine kinase – Role and significance in Cancer. International Journal of Medical Sciences 2004, 1, 101-115, 10.7150/ijms.1.101.
- Srinivasan Madhusudan; Trivadi S. Ganesan; Tyrosine kinase inhibitors in cancer therapy. Clinical Biochemistry 2004, 37, 618-635, 10.1016/j.clinbiochem.2004.05.006.
- Monique Nilsson; John V. Heymach; Vascular Endothelial Growth Factor (VEGF) Pathway. Journal of Thoracic Oncology 2006, 1, 768-770, 10.1016/s1556-0864(15)30404-4.
- Yixiao Feng; Mia Spezia; Shifeng Huang; Chengfu Yuan; Zongyue Zeng; Linghuan Zhang; Xiaojuan Ji; Wei Liu; Bo Huang; Wenping Luo; et al. Breast cancer development and progression: Risk factors, cancer stem cells, signaling pathways, genomics, and molecular pathogenesis. Genes & Diseases 2018, 5, 77-106, 10.1016/j.gendis.2018.05.001.
- Lihua Huang; Liwu Fu; Mechanisms of resistance to EGFR tyrosine kinase inhibitors. Acta Pharmaceutica Sinica B 2015, 5, 390-401, 10.1016/j.apsb.2015.07.001.
- N. A. Seebacher; A. E. Stacy; G. M. Porter; A. M. Merlot; Clinical development of targeted and immune based anti-cancer therapies. Journal of Experimental & Clinical Cancer Research 2019, 38, 1-39, 10.1186/s13046-019-1094-2.
- Laura Pacini; Andrew Jenks; Nadia Lima; Paul Huang; Targeting the Fibroblast Growth Factor Receptor (FGFR) Family in Lung Cancer. Cells 2021, 10, 1154, 10.3390/cells10051154.
- Alberto Puccini; Nagore I. Marín-Ramos; Francesca Bergamo; Marta Schirripa; Sara Lonardi; Heinz-Josef Lenz; Fotios Loupakis; Francesca Battaglin; Safety and Tolerability of c-MET Inhibitors in Cancer. Drug Safety 2019, 42, 211-233, 10.1007/s40264-018-0780-x.
- Priscilla Cascetta; Vincenzo Sforza; Anna Manzo; Guido Carillio; Giuliano Palumbo; Giovanna Esposito; Agnese Montanino; Raffaele Costanzo; Claudia Sandomenico; Rossella De Cecio; et al. RET Inhibitors in Non-Small-Cell Lung Cancer. Cancers 2021, 13, 4415, 10.3390/cancers13174415.
- Julia Rotow; Trever G. Bivona; Understanding and targeting resistance mechanisms in NSCLC. Nature Reviews Cancer 2017, 17, 637-658, 10.1038/nrc.2017.84.
- Luc Friboulet; Nanxin Li; Ryohei Katayama; Christian C. Lee; Justin F. Gainor; Adam Crystal; Pierre-Yves Michellys; Mark M. Awad; Noriko Yanagitani; Sungjoon Kim; et al. The ALK Inhibitor Ceritinib Overcomes Crizotinib Resistance in Non–Small Cell Lung Cancer. Cancer Discovery 2014, 4, 662-673, 10.1158/2159-8290.cd-13-0846.
- ZYKADIA (ceritinib). Prescribing information. . U.S Food & Drug Administration. Retrieved 2022-4-21
- Elena Ardini; Maria Menichincheri; Patrizia Banfi; Roberta Bosotti; Cristina De Ponti; Romana Pulci; Dario Ballinari; Marina Ciomei; Gemma Texido; Anna Degrassi; et al. Entrectinib, a Pan–TRK, ROS1, and ALK Inhibitor with Activity in Multiple Molecularly Defined Cancer Indications. Molecular Cancer Therapeutics 2016, 15, 628-639, 10.1158/1535-7163.mct-15-0758.
- Suresh S. Ramalingam; Johan Vansteenkiste; David Planchard; Byoung Chul Cho; Jhanelle E. Gray; Yuichiro Ohe; Caicun Zhou; Thanyanan Reungwetwattana; Ying Cheng; Busyamas Chewaskulyong; et al. Overall Survival with Osimertinib in Untreated, EGFR-Mutated Advanced NSCLC. New England Journal of Medicine 2020, 382, 41-50, 10.1056/nejmoa1913662.
- Wenjun Zhou; Dalia Ercan; Liang Chen; Cai-Hong Yun; Danan Li; Marzia Capelletti; Alexis Cortot; Lucian Chirieac; Roxana E. Iacob; Robert Padera; et al. Novel mutant-selective EGFR kinase inhibitors against EGFR T790M. Nature 2009, 462, 1070-1074, 10.1038/nature08622.
- Francois Gonzalvez; Sylvie Vincent; Theresa E. Baker; Alexandra E. Gould; Shuai Li; Scott D. Wardwell; Sara Nadworny; Yaoyu Ning; Sen Zhang; Wei-Sheng Huang; et al. Mobocertinib (TAK-788): A Targeted Inhibitor of EGFR Exon 20 Insertion Mutants in Non–Small Cell Lung Cancer. Cancer Discovery 2021, 11, 1672-1687, 10.1158/2159-8290.cd-20-1683.
- Johan Filip Vansteenkiste; Charlotte Van De Kerkhove; Els Wauters; Pierre Van Mol; Capmatinib for the treatment of non-small cell lung cancer. Expert Review of Anticancer Therapy 2019, 19, 659-671, 10.1080/14737140.2019.1643239.
- Paul K. Paik; Enriqueta Felip; Remi Veillon; Hiroshi Sakai; Alexis B. Cortot; Marina C. Garassino; Julien Mazieres; Santiago Viteri; Helene Senellart; Jan Van Meerbeeck; et al. Tepotinib in Non–Small-Cell Lung Cancer with MET Exon 14 Skipping Mutations. New England Journal of Medicine 2020, 383, 931-943, 10.1056/nejmoa2004407.
- NERLYNX (neratinib). Prescribing Information. . U.S Food & Drug Administration. Retrieved 2022-4-21
- TUKYSA (tucatinib). Prescribing Information. . U.S Food & Drug Administration. Retrieved 2022-4-21
- Cristina Saura; Mafalda Oliveira; Yin-Hsun Feng; Ming-Shen Dai; Shang-Wen Chen; Sara A. Hurvitz; Sung-Bae Kim; Beverly Moy; Suzette Delaloge; William Gradishar; et al. Neratinib Plus Capecitabine Versus Lapatinib Plus Capecitabine in HER2-Positive Metastatic Breast Cancer Previously Treated With ≥ 2 HER2-Directed Regimens: Phase III NALA Trial. Journal of Clinical Oncology 2020, 38, 3138-3149, 10.1200/jco.20.00147.
- Rashmi K. Murthy; Sherene Loi; Alicia Okines; Elisavet Paplomata; Erika Hamilton; Sara A. Hurvitz; Nancy U. Lin; Virginia Borges; Vandana Abramson; Carey Anders; et al. Tucatinib, Trastuzumab, and Capecitabine for HER2-Positive Metastatic Breast Cancer. New England Journal of Medicine 2020, 382, 597-609, 10.1056/nejmoa1914609.
- Anita Kulukian; Patrice Lee; Janelle Taylor; Robert Rosler; Peter De Vries; Daniel Watson; Andres Forero-Torres; Scott Peterson; Preclinical Activity of HER2-Selective Tyrosine Kinase Inhibitor Tucatinib as a Single Agent or in Combination with Trastuzumab or Docetaxel in Solid Tumor Models. Molecular Cancer Therapeutics 2020, 19, 976-987, 10.1158/1535-7163.mct-19-0873.
- Sridhar K. Rabindran; Carolyn M. Discafani; Edward Rosfjord; Michelle Baxter; M. Brawner Floyd; Jonathan Golas; William A. Hallett; Bernard D. Johnson; Ramaswamy Nilakantan; Elsebe Overbeek; et al. Antitumor Activity of HKI-272, an Orally Active, Irreversible Inhibitor of the HER-2 Tyrosine Kinase. Cancer Research 2004, 64, 3958-3965, 10.1158/0008-5472.can-03-2868.
- Ghassan K Abou-Alfa; Vaibhav Sahai; Antoine Hollebecque; Gina Vaccaro; Davide Melisi; Raed Al-Rajabi; Andrew S Paulson; Mitesh J Borad; David Gallinson; Adrian G Murphy; et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: a multicentre, open-label, phase 2 study. The Lancet Oncology 2020, 21, 671-684, 10.1016/s1470-2045(20)30109-1.
- Milind Javle; Sameek Roychowdhury; Robin Kate Kelley; Saeed Sadeghi; Teresa Macarulla; Karl Heinz Weiss; Dirk-Thomas Waldschmidt; Lipika Goyal; Ivan Borbath; Anthony El-Khoueiry; et al. Infigratinib (BGJ398) in previously treated patients with advanced or metastatic cholangiocarcinoma with FGFR2 fusions or rearrangements: mature results from a multicentre, open-label, single-arm, phase 2 study. The Lancet Gastroenterology & Hepatology 2021, 6, 803-815, 10.1016/s2468-1253(21)00196-5.
- PEMAZYRE (pemigatinib). Prescribing information. . U.S Food & Drug Administration. Retrieved 2022-4-21
- TRUSELTIQ (infigratinib). Prescribing information. . U.S Food & Drug Administration. Retrieved 2022-4-21
- Saadia A Aziz; Joshua Sznol; Adebowale Adeniran; John W Colberg; Robert L Camp; Harriet M Kluger; Vascularity of primary and metastatic renal cell carcinoma specimens. Journal of Translational Medicine 2013, 11, 15-15, 10.1186/1479-5876-11-15.
- Muhammad Omer Jamil; Amanda Hathaway; AmitKumar Mehta; Tivozanib: Status of Development. Current Oncology Reports 2015, 17, 1-7, 10.1007/s11912-015-0451-3.
- Brian I Rini; Sumanta K Pal; Bernard J Escudier; Michael B Atkins; Thomas Hutson; Camillo Porta; Elena Verzoni; Michael N Needle; David F McDermott; Tivozanib versus sorafenib in patients with advanced renal cell carcinoma (TIVO-3): a phase 3, multicentre, randomised, controlled, open-label study. The Lancet Oncology 2019, 21, 95-104, 10.1016/s1470-2045(19)30735-1.
- FOTIVDA (tivozanib). Prescribing information. . Aveo Oncology. Retrieved 2022-4-21
- Anthony Markham; Mobocertinib: First Approval. Drugs 2021, 81, 2069-2074, 10.1007/s40265-021-01632-9.
- Connie Kang; Infigratinib: First Approval. Drugs 2021, 81, 1355-1360, 10.1007/s40265-021-01567-1.
- Infigratinib for the treatment of cholangiocarcinoma. . European Medicines Agency.. Retrieved 2022-4-21
- Esther S. Kim; Tivozanib: First Global Approval. Drugs 2017, 77, 1917-1923, 10.1007/s40265-017-0825-y.
- Fotivda: EPAR - Product Information. . European Medicines Agency. Retrieved 2022-4-21
- Anthony Markham; Tepotinib: First Approval. Drugs 2020, 80, 829-833, 10.1007/s40265-020-01317-9.
- Anthony Markham; Pralsetinib: First Approval. Drugs 2020, 80, 1865-1870, 10.1007/s40265-020-01427-4.
- Gavreto: EPAR – Medicine Overview. . European Medicines Agency. Retrieved 2022-4-21
- Sohita Dhillon; Capmatinib: First Approval. Drugs 2020, 80, 1125-1131, 10.1007/s40265-020-01347-3.
- Sheridan M. Hoy; Pemigatinib: First Approval. Drugs 2020, 80, 923-929, 10.1007/s40265-020-01330-y.
- Pemazyre: EPAR - Product Information. . European Medicines Agency. Retrieved 2022-4-21
- Arnold Lee; Tucatinib: First Approval. Drugs 2020, 80, 1033-1038, 10.1007/s40265-020-01340-w.
- Tukysa: EPAR – Medicine Overview. . European Medicines Agency. Retrieved 2022-4-21
- Emma D. Deeks; Neratinib: First Global Approval. Drugs 2017, 77, 1695-1704, 10.1007/s40265-017-0811-4.
- Nerlynx: EPAR – Medicine Overview. . European Medicines Agency. Retrieved 2022-4-21
- Sarah L. Greig; Osimertinib: First Global Approval. Drugs 2016, 76, 263-273, 10.1007/s40265-015-0533-4.
- Tagrisso: EPAR – Medicine Overview. . European Medicines Agency. Retrieved 2022-4-21
- Sohita Dhillon; Madeleine Clark; Ceritinib: First Global Approval. Drugs 2014, 74, 1285-1291, 10.1007/s40265-014-0251-3.
- Zykadia: EPAR – Medicine Overview. . European Medicines Agency. Retrieved 2022-4-21
- Robert Roskoski; Cyclin-dependent protein serine/threonine kinase inhibitors as anticancer drugs. Pharmacological Research 2018, 139, 471-488, 10.1016/j.phrs.2018.11.035.
- Concepción Sánchez-Martínez; María José Lallena; Sonia Gutiérrez Sanfeliciano; Alfonso de Dios; Cyclin dependent kinase (CDK) inhibitors as anticancer drugs: Recent advances (2015–2019). Bioorganic & Medicinal Chemistry Letters 2019, 29, 126637, 10.1016/j.bmcl.2019.126637.
- Francesco Schettini; Irene De Santo; Carmen G. Rea; Pietro De Placido; Luigi Formisano; Mario Giuliano; Grazia Arpino; Michelino De Laurentiis; Fabio Puglisi; Sabino De Placido; et al. CDK 4/6 Inhibitors as Single Agent in Advanced Solid Tumors. Frontiers in Oncology 2018, 8, 608, 10.3389/fonc.2018.00608.
- Julia A. Beaver; Laleh Amiri-Kordestani; Rosane Charlab; Wei Chen; Todd Palmby; Amy Tilley; Jeanne Fourie Zirkelbach; Jingyu Yu; Qi Liu; Liang Zhao; et al. FDA Approval: Palbociclib for the Treatment of Postmenopausal Patients with Estrogen Receptor–Positive, HER2-Negative Metastatic Breast Cancer. Clinical Cancer Research 2015, 21, 4760-4766, 10.1158/1078-0432.ccr-15-1185.
- Amanda J. Walker; Suparna Wedam; Laleh Amiri-Kordestani; Erik Bloomquist; Shenghui Tang; Rajeshwari Sridhara; Wei Chen; Todd R. Palmby; Jeanne Fourie Zirkelbach; Wentao Fu; et al. FDA Approval of Palbociclib in Combination with Fulvestrant for the Treatment of Hormone Receptor–Positive, HER2-Negative Metastatic Breast Cancer. Clinical Cancer Research 2016, 22, 4968-4972, 10.1158/1078-0432.ccr-16-0493.
- Joyce O’Shaughnessy; Katarina Petrakova; Gabe S. Sonke; Pierfranco Conte; Carlos L. Arteaga; David A. Cameron; Lowell L. Hart; Cristian Villanueva; Erik Jakobsen; Joseph T. Beck; et al. Ribociclib plus letrozole versus letrozole alone in patients with de novo HR+, HER2− advanced breast cancer in the randomized MONALEESA-2 trial. Breast Cancer Research and Treatment 2017, 168, 127-134, 10.1007/s10549-017-4518-8.
- Amita Patnaik; Lee S. Rosen; Sara M. Tolaney; Anthony W. Tolcher; Jonathan W. Goldman; Leena Gandhi; Kyriakos P. Papadopoulos; Muralidhar Beeram; Drew W. Rasco; John F. Hilton; et al. Efficacy and Safety of Abemaciclib, an Inhibitor of CDK4 and CDK6, for Patients with Breast Cancer, Non–Small Cell Lung Cancer, and Other Solid Tumors. Cancer Discovery 2016, 6, 740-753, 10.1158/2159-8290.cd-16-0095.
- Thomas J. Raub; Graham N. Wishart; Palaniappan Kulanthaivel; Brian A. Staton; Rose T. Ajamie; Geri A. Sawada; Lawrence M. Gelbert; Harlan E. Shannon; Concepcion Sanchez-Martinez; Alfonso De Dios; et al. Brain Exposure of Two Selective Dual CDK4 and CDK6 Inhibitors and the Antitumor Activity of CDK4 and CDK6 Inhibition in Combination with Temozolomide in an Intracranial Glioblastoma Xenograft. Drug Metabolism and Disposition 2015, 43, 1360-1371, 10.1124/dmd.114.062745.
- Sara M. Tolaney; Solmaz Sahebjam; Emilie Le Rhun; Thomas Bachelot; Peter Kabos; Ahmad Awada; Denise Yardley; Arlene Chan; Pierfranco Conte; Véronique Diéras; et al. A Phase II Study of Abemaciclib in Patients with Brain Metastases Secondary to Hormone Receptor–Positive Breast Cancer. Clinical Cancer Research 2020, 26, 5310-5319, 10.1158/1078-0432.ccr-20-1764.
- Esther S. Kim; Abemaciclib: First Global Approval. Drugs 2017, 77, 2063-2070, 10.1007/s40265-017-0840-z.
- Verzenios: EPAR – Medicine Overview. . European Medicines Agency. Retrieved 2022-4-21
- Yahiya Y. Syed; Ribociclib: First Global Approval. Drugs 2017, 77, 799-807, 10.1007/s40265-017-0742-0.
- Kisqali: EPAR – Medicine Overview. . European Medicines Agency. Retrieved 2022-4-21
- Sohita Dhillon; Palbociclib: First Global Approval. Drugs 2015, 75, 543-551, 10.1007/s40265-015-0379-9.
- Ibrance: EPAR - Product Information. . European Medicines Agency. Retrieved 2022-4-21
- Martin Krug; Recent Advances in the Development of Multi-Kinase Inhibitors. Mini-Reviews in Medicinal Chemistry 2008, 8, 1312-1327, 10.2174/138955708786369591.
- Fleur Broekman; Tyrosine kinase inhibitors: Multi-targeted or single-targeted?. World Journal of Clinical Oncology 2011, 2, 80-93, 10.5306/wjco.v2.i2.80.
- Elena Ardini; Maria Menichincheri; Patrizia Banfi; Roberta Bosotti; Cristina De Ponti; Romana Pulci; Dario Ballinari; Marina Ciomei; Gemma Texido; Anna Degrassi; et al. Entrectinib, a Pan–TRK, ROS1, and ALK Inhibitor with Activity in Multiple Molecularly Defined Cancer Indications. Molecular Cancer Therapeutics 2016, 15, 628-639, 10.1158/1535-7163.mct-15-0758.
- Shouzheng Wang; Junling Li; Second-generation EGFR and ErbB tyrosine kinase inhibitors as first-line treatments for non-small cell lung cancer.. OncoTargets and Therapy 2019, ume 12, 6535-6548, 10.2147/OTT.S198945.
- Yohann Loriot; Andrea Necchi; Se Hoon Park; Jesus Garcia-Donas; Robert Huddart; Earle Burgess; Mark Fleming; Arash Rezazadeh; Begoña Mellado; Sergey Varlamov; et al. Erdafitinib in Locally Advanced or Metastatic Urothelial Carcinoma. New England Journal of Medicine 2019, 381, 338-348, 10.1056/nejmoa1817323.
- Anthony Markham; Erdafitinib: First Global Approval. Drugs 2019, 79, 1017-1021, 10.1007/s40265-019-01142-9.
- William D. Tap; Zev A. Wainberg; Stephen P. Anthony; Prabha N. Ibrahim; Chao Zhang; John Healey; Bartosz Chmielowski; Arthur P. Staddon; Allen Lee Cohn; Geoffrey I. Shapiro; et al. Structure-Guided Blockade of CSF1R Kinase in Tenosynovial Giant-Cell Tumor. New England Journal of Medicine 2015, 373, 428-437, 10.1056/nejmoa1411366.
- Rajmohan Murali; Alexander M Menzies; Gv Long; Dabrafenib and its potential for the treatment of metastatic melanoma. Drug Design, Development and Therapy 2012, 6, 391-405, 10.2147/dddt.s38998.
- James Sun; Jonathan S. Zager; Zeynep Eroglu; Encorafenib/binimetinib for the treatment of BRAF-mutant advanced, unresectable, or metastatic melanoma: design, development, and potential place in therapy. OncoTargets and Therapy 2018, ume 11, 9081-9089, 10.2147/ott.s171693.
- Reinhard Dummer; Paolo A Ascierto; Helen J Gogas; Ana Arance; Mario Mandala; Gabriella Liszkay; Claus Garbe; Dirk Schadendorf; Ivana Krajsova; Ralf Gutzmer; et al. Encorafenib plus binimetinib versus vemurafenib or encorafenib in patients with BRAF -mutant melanoma (COLUMBUS): a multicentre, open-label, randomised phase 3 trial. The Lancet Oncology 2018, 19, 603-615, 10.1016/s1470-2045(18)30142-6.
- Robert Roskoski; Targeting oncogenic Raf protein-serine/threonine kinases in human cancers. Pharmacological Research 2018, 135, 239-258, 10.1016/j.phrs.2018.08.013.
- Andrea M. Gross; Pamela L. Wolters; Eva Dombi; Andrea Baldwin; Patricia Whitcomb; Michael J. Fisher; Brian Weiss; AeRang Kim; Miriam Bornhorst; Amish C. Shah; et al. Selumetinib in Children with Inoperable Plexiform Neurofibromas. New England Journal of Medicine 2020, 382, 1430-1442, 10.1056/nejmoa1912735.
- Scott Kopetz; Axel Grothey; Rona Yaeger; Eric Van Cutsem; Jayesh Desai; Takayuki Yoshino; Harpreet Wasan; Fortunato Ciardiello; Fotios Loupakis; Yong Sang Hong; et al. Encorafenib, Binimetinib, and Cetuximab in BRAF V600E–Mutated Colorectal Cancer. New England Journal of Medicine 2019, 381, 1632-1643, 10.1056/nejmoa1908075.
- Dirk Strumberg; Beate Schultheis; Regorafenib for cancer. Expert Opinion on Investigational Drugs 2012, 21, 879-889, 10.1517/13543784.2012.684752.
- Jean-Yves Blay; César Serrano; Michael C Heinrich; John Zalcberg; Sebastian Bauer; Hans Gelderblom; Patrick Schöffski; Robin L Jones; Steven Attia; Gina D'Amato; et al. Ripretinib in patients with advanced gastrointestinal stromal tumours (INVICTUS): a double-blind, randomised, placebo-controlled, phase 3 trial. The Lancet Oncology 2020, 21, 923-934, 10.1016/s1470-2045(20)30168-6.
- Bryan D. Smith; Michael D. Kaufman; Wei-Ping Lu; Anu Gupta; Cynthia B. Leary; Scott C. Wise; Thomas J. Rutkoski; Yu Mi Ahn; Gada Al-Ani; Stacie L. Bulfer; et al. Ripretinib (DCC-2618) Is a Switch Control Kinase Inhibitor of a Broad Spectrum of Oncogenic and Drug-Resistant KIT and PDGFRA Variants. Cancer Cell 2019, 35, 738-751.e9, 10.1016/j.ccell.2019.04.006.
- Maria E Cabanillas; Mabel Ryder; Camilo Jimenez; Targeted Therapy for Advanced Thyroid Cancer: Kinase Inhibitors and Beyond. Endocrine Reviews 2019, 40, 1573-1604, 10.1210/er.2019-00007.
- Lori J. Wirth; Eric Sherman; Bruce Robinson; Benjamin Solomon; Hyunseok Kang; Jochen Lorch; Francis Worden; Marcia Brose; Jyoti Patel; Sophie Leboulleux; et al. Efficacy of Selpercatinib in RET-Altered Thyroid Cancers. New England Journal of Medicine 2020, 383, 825-835, 10.1056/nejmoa2005651.
- Vivek Subbiah; Robert J. Kreitman; Zev A. Wainberg; Jae Yong Cho; Jan H.M. Schellens; Jean Charles Soria; Patrick Y. Wen; Christoph Zielinski; Maria E. Cabanillas; Gladys Urbanowitz; et al. Dabrafenib and Trametinib Treatment in Patients With Locally Advanced or Metastatic BRAF V600–Mutant Anaplastic Thyroid Cancer. Journal of Clinical Oncology 2018, 36, 7-13, 10.1200/jco.2017.73.6785.
- Theodore W. Laetsch; Douglas S. Hawkins; Larotrectinib for the treatment of TRK fusion solid tumors. Expert Review of Anticancer Therapy 2018, 19, 1-10, 10.1080/14737140.2019.1538796.
- Stephanie Berger; Uwe M. Martens; Sylvia Bochum; Larotrectinib (LOXO-101). Computational Biology 2018, 211, 141-151, 10.1007/978-3-319-91442-8_10.
- Alexander Drilon; Salvatore Siena; Sai-Hong Ignatius Ou; Manish Patel; Myung Ju Ahn; Jeeyun Lee; Todd M. Bauer; Anna F. Farago; Jennifer J. Wheler; Stephen V. Liu; et al. Safety and Antitumor Activity of the Multitargeted Pan-TRK, ROS1, and ALK Inhibitor Entrectinib: Combined Results from Two Phase I Trials (ALKA-372-001 and STARTRK-1). Cancer Discovery 2017, 7, 400-409, 10.1158/2159-8290.cd-16-1237.
- Maria E. Cabanillas; Mouhammed Amir Habra; Lenvatinib: Role in thyroid cancer and other solid tumors. Cancer Treatment Reviews 2015, 42, 47-55, 10.1016/j.ctrv.2015.11.003.
- Vincent Chau; Marijo Bilusic; Pembrolizumab in Combination with Axitinib as First-Line Treatment for Patients with Renal Cell Carcinoma (RCC): Evidence to Date. Cancer Management and Research 2020, ume 12, 7321-7330, 10.2147/cmar.s216605.
- Toni K Choueiri; James Larkin; Mototsugu Oya; Fiona Thistlethwaite; Marcella Martignoni; Paul Nathan; Thomas Powles; David McDermott; Paul B Robbins; David D Chism; et al. Preliminary results for avelumab plus axitinib as first-line therapy in patients with advanced clear-cell renal-cell carcinoma (JAVELIN Renal 100): an open-label, dose-finding and dose-expansion, phase 1b trial. The Lancet Oncology 2018, 19, 451-460, 10.1016/s1470-2045(18)30107-4.
- Guru Sonpavde; Thomas Hutson; Brian I Rini; Axitinib for renal cell carcinoma. Expert Opinion on Investigational Drugs 2008, 17, 741-748, 10.1517/13543784.17.5.741.
- Sohita Dhillon; Ripretinib: First Approval. Drugs 2020, 80, 1133-1138, 10.1007/s40265-020-01348-2.
- Qinlock: EPAR – Medicine Overview. . European Medicines Agency. Retrieved 2022-4-21
- Anthony Markham; Selpercatinib: First Approval. Drugs 2020, 80, 1119-1124, 10.1007/s40265-020-01343-7.
- Retsevmo: EPAR – Medicine Overview. . European Medicines Agency. Retrieved 2022-4-21
- Anthony Markham; Susan J. Keam; Selumetinib: First Approval. Drugs 2020, 80, 931-937, 10.1007/s40265-020-01331-x.
- Koselugo: EPAR – Medicine Overview. . European Medicines Agency. Retrieved 2022-4-21
- Sohita Dhillon; Avapritinib: First Approval. Drugs 2020, 80, 433-439, 10.1007/s40265-020-01275-2.
- Ayvakyt: EPAR – Medicine Overview. . European Medicines Agency. Retrieved 2022-4-21
- Zaina T. Al-Salama; Susan J. Keam; Entrectinib: First Global Approval. Drugs 2019, 79, 1477-1483, 10.1007/s40265-019-01177-y.
- Rozlytrek: EPAR – Overview. . European Medicines Agency. Retrieved 2022-4-21
- Yvette N. Lamb; Pexidartinib: First Approval. Drugs 2019, 79, 1805-1812, 10.1007/s40265-019-01210-0.
- Refusal of the marketing authorisation for Turalio (pexidartinib). . European Medicines Agency. Retrieved 2022-4-21
- Lesley J. Scott; Larotrectinib: First Global Approval. Drugs 2019, 79, 201-206, 10.1007/s40265-018-1044-x.
- Vitrakvi: EPAR - Product Information. . European Medicines Agency. Retrieved 2022-4-21
- Yahiya Y. Syed; Lorlatinib: First Global Approval. Drugs 2019, 79, 93-98, 10.1007/s40265-018-1041-0.
- Lorviqua: EPAR – Medicine Overview. . European Medicines Agency. Retrieved 2022-4-21
- Matt Shirley; Dacomitinib: First Global Approval. Drugs 2018, 78, 1947-1953, 10.1007/s40265-018-1028-x.
- Vizimpro: EPAR - Product Information. . European Medicines Agency. Retrieved 2022-4-21
- Matt Shirley; Encorafenib and Binimetinib: First Global Approvals. Drugs 2018, 78, 1277-1284, 10.1007/s40265-018-0963-x.
- Braftovi: EPAR – Medicine Overview. . European Medicines Agency. Retrieved 2022-4-21
- Mektovi: EPAR – Medicine Overview. . European Medicines Agency. Retrieved 2022-4-21
- Anthony Markham; Brigatinib: First Global Approval. Drugs 2017, 77, 1131-1135, 10.1007/s40265-017-0776-3.
- Alunbrig: EPAR - Product Information. . European Medicines Agency. Retrieved 2022-4-21
- Julia Paik; Sohita Dhillon; Alectinib: A Review in Advanced, ALK-Positive NSCLC. Drugs 2018, 78, 1247-1257, 10.1007/s40265-018-0952-0.
- Alecensa: EPAR – Summary for the public. . European Medicines Agency. Retrieved 2022-4-21
- Karly P. Garnock-Jones; Cobimetinib: First Global Approval. Drugs 2015, 75, 1823-1830, 10.1007/s40265-015-0477-8.
- Cotellic: EPAR – Summary for the public. . European Medicines Agency. Retrieved 2022-4-21
- Lesley J. Scott; Lenvatinib: First Global Approval. Drugs 2015, 75, 553-560, 10.1007/s40265-015-0383-0.
- Lenvima: EPAR – Medicine Overview. . European Medicines Agency. Retrieved 2022-4-21
- Rosselle T. Dungo; Gillian M. Keating; Afatinib: First Global Approval. Drugs 2013, 73, 1503-1515, 10.1007/s40265-013-0111-6.
- Giotrif: EPAR – Summary for the public. . European Medicines Agency. Retrieved 2022-4-21
- Cameron J. M. Wright; Paul L. McCormack; Trametinib: First Global Approval. Drugs 2013, 73, 1245-1254, 10.1007/s40265-013-0096-1.
- Mekinist: EPAR – Medicine Overview. . European Medicines Agency. Retrieved 2022-4-21
- Anita D. Ballantyne; Karly P. Garnock-Jones; Dabrafenib: First Global Approval. Drugs 2013, 73, 1367-1376, 10.1007/s40265-013-0095-2.
- Tafinlar: EPAR – Medicine Overview. . European Medicines Agency. Retrieved 2022-4-21
- Harpreet Singh; Michael Brave; Julia A. Beaver; Joyce Cheng; Shenghui Tang; Eias Zahalka; Todd R. Palmby; Rajesh Venugopal; Pengfei Song; Qi Liu; et al. U.S. Food and Drug Administration Approval: Cabozantinib for the Treatment of Advanced Renal Cell Carcinoma. Clinical Cancer Research 2016, 23, 330-335, 10.1158/1078-0432.ccr-16-1073.
- CABOMETYX (cabozantinib). Prescribing Information. . U.S Food & Drug Administration. Retrieved 2022-4-21
- Cabometyx: EPAR – Medicine Overview. . European Medicines Agency. Retrieved 2022-4-21
- Subhajit Roy; Bawneet K Narang; Shiva K Rastogi; Ravindra K Rawal; A Novel Multiple Tyrosine-kinase Targeted Agent to Explore the Future Perspectives of Anti-Angiogenic Therapy for the Treatment of Multiple Solid Tumors: Cabozantinib. Anti-Cancer Agents in Medicinal Chemistry 2014, 15, 37-47, 10.2174/1871520614666140902153840.
- COMETRIQ (cabozantinib). Prescribing Information. . U.S Food & Drug Administration. Retrieved 2022-4-21
- Cometriq: EPAR – Summary for the public. . European Medicines Agency. Retrieved 2022-4-21
- Thomas J. Ettrich; Thomas Seufferlein; Regorafenib. Methods in Pharmacology and Toxicology 2014, 201, 185-196, 10.1007/978-3-642-54490-3_10.
- STIVARGA (regorafenib). Prescribing Information. . U.S Food & Drug Administration. Retrieved 2022-4-21
- Stivarga: EPAR – Summary for the public. . European Medicines Agency. Retrieved 2022-4-21
- Audrey Bellesoeur; Edith Carton; Jerome Alexandre; Francois Goldwasser; Olivier Huillard; Axitinib in the treatment of renal cell carcinoma: design, development, and place in therapy. Drug Design, Development and Therapy 2017, ume 11, 2801-2811, 10.2147/dddt.s109640.
- INLYTA (axitinib). Prescribing Information. . U.S Food & Drug Administration. Retrieved 2022-4-21
- Inlyta: EPAR – Summary for the public. . European Medicines Agency. Retrieved 2022-4-21
- Alice T. Shaw; Uma Yasothan; Peter Kirkpatrick; Crizotinib. Nature Reviews Drug Discovery 2011, 10, 897-898, 10.1038/nrd3600.
- XALKORI (crizotinib). Prescribing Information. . U.S Food & Drug Administration. Retrieved 2022-4-21
- Xalkori: EPAR – Summary for the public. . European Medicines Agency. Retrieved 2022-4-21
- Keith. T Flaherty; Uma Yasothan; Peter Kirkpatrick; Vemurafenib. Nature Reviews Drug Discovery 2011, 10, 811-812, 10.1038/nrd3579.
- ZELBORAF (vemurafenib). Prescribing Information. . U.S Food & Drug Administration. Retrieved 2022-4-21
- Zelboraf: EPAR – Summary for the public. . European Medicines Agency. Retrieved 2022-4-21
- James E. Frampton; Vandetanib. Drugs 2012, 72, 1423-1436, 10.2165/11209300-000000000-00000.
- Caprelsa: EPAR – Summary for the public. . European Medicines Agency. Retrieved 2022-4-21
This entry is offline, you can click here to edit this entry!
