2. Surface
2.1. Anatomy and Key Cells
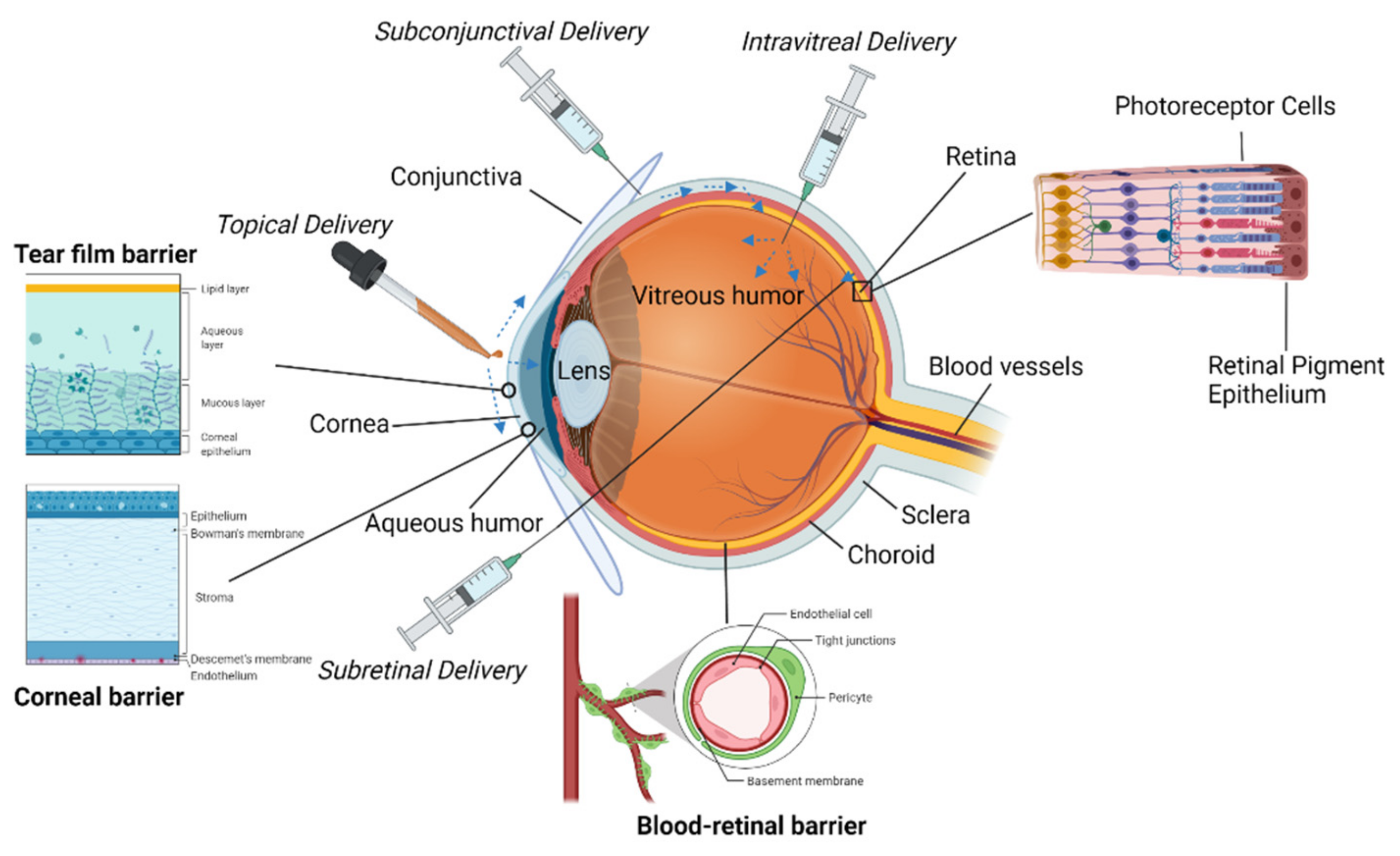
The surface of the eye has three primary components: the cornea, the conjunctiva, and a protective tear film
[1]. Despite their seeming simplicity, this portion of the eye not only is extremely important for vision, but also plays a role in the body’s innate immune system.
2.1.1. The Cornea
The cornea is a highly specialized tissue that serves as a mechanical barrier to prevent microorganisms from entering the eye
[2]. Along with the tear film, it is also responsible for the refraction of light as it enters the eye. The cornea is covered in a nonkeratinized stratified squamous epithelium that consists of 5–7 layers of cells
[2]. These cells also are connected by tight junctions to keep liquid, toxins, or microbes from entering the intracellular spaces
[3]. The cells of the epithelium lie on a basement membrane made primarily of type IV collagen and laminin
[3]. Following the epithelium is the Bowman layer or Bowman membrane which is an acellular condensate. This layer will not regenerate if damaged; however, it has been known to scar
[3].
Next is the corneal stroma, which provides the structural support for the cornea and most of its thickness. The stroma relies on the precise organization of both the stromal fibers and extracellular matrix for transparency
[3]. Keratocytes, generally located in the anterior of the stroma, are the primary cell type and comprise the extracellular matrix found there
[3]. A unique characteristic of these cells is that they contain corneal “crystallins”, which are made up of soluble proteins and play a role in reducing the backscatter of light due to the keratocytes
[3]. This backscatter reduction is an important part maintaining cornea transparency
[3]. The next layer is the Descemet membrane, which is continuously secreted by endothelial cells
[3]. The parts of the membrane produced after birth are unbanded and amorphous in structure, while the portions formed
in utero have a distinctive banding pattern
[3].
The final layer is the corneal endothelium which maintains the deturgescence, or dehydrated, state of the cornea, which is necessary for clear vision. The endothelial cells here are initially hexagonal; however, as the cell number drops due to age, trauma, or inflammation, the remaining endothelial cells can stretch to cover gaps that emerge. This stretching causes the cells to grow and lose their hexagonal shape
[3]. This is believed to be due to the cornea’s need to maintain a stable metabolic state. As mentioned already, the endothelium is responsible for maintaining the state of the cornea, which includes controlling metabolic inputs and outputs.When cells die, the remaining cells must assume their duties, causing them to take over the functions of the degenerated cells
[3]. It should also be noted that corneal metabolism depends heavily on a critical oxygen level and exposure to low oxygen levels may cause permanent morphological changes to the corneal epithelium in addition to damage to the overall corneal physiology
[4].
The cornea connects to the conjunctiva at the corneoscleral junction or limbus
[2]. The limbus works with the conjunctiva to support the cornea and ensures the conjunctiva does not grow into the cornea. The epithelium of the limbus is continuous with the epithelium of the cornea, making its boundaries difficult to define
[2]. The limbus is composed of a nonkeratinized stratified limbal epithelium; however, unlike the conjunctival epithelium, it lacks goblet cells
[2].
The limbal epithelium has several layers and contains mature epithelial dendritic cells, immature epithelial dendritic cells, T lymphocytes, and pigmented melanocytes. The basal layer of the limbus contains limbal epithelial stem cells (LESCs), which produce the corneal epithelium
[3]. LESCs are located in what is called the limbal niche and are capable of two types of division
[2]. During symmetric division, LESCs produce either two new stem cells or two new daughter cells; during asymmetric division, they produce a stem cell and an early transient amplifying cell (eTAC) which can then divide and give rise to the transient amplifying cells (TACs). TACs can migrate and terminally differentiate into corneal epithelial cells
[2]. The limbal niche is both vascularized and innervated, unlike the cornea.
2.1.2. The Conjunctiva
The conjunctiva is located on the surface of the eye and the posterior of the eyelids. It is composed of several parts that together with the surface of the cornea are referred to as the conjunctival sac
[5]. The bulbar conjunctiva covers the white portion of the eye that is visible or exposed. The palpebral conjunctiva is located on the posterior of the eyelids
[5]. The conjunctival fornix (or forniceal conjunctiva) connects the bulbar and palpebral conjunctiva. As a tissue, the conjunctiva is primarily responsible for lubricating the eye
[5]; however, it also serves to protect the soft tissues located in the eye, supply immune tissue, and allow the eye to move
[6]. The epithelial cells of the conjunctiva contain microvilli which are an important part of allowing the tear film to adhere to the surface of the eye
[6]. The conjunctiva also contains a part of the mucosa-associated lymphoid tissue (MALT) called the conjunctiva-associated lymphoid tissue (CALT). This system has all the components needed for a complete immune response
[6]. This system can help a tissue introduce tolerance to some antigens, as well as detect antigens, and induce a direct immune response. In addition, the conjunctiva secretes IgA as well as some other components of the secretory immune system
[7].
On the cellular level, the bulbar conjunctiva is mostly nonkeratinizing squamous epithelium. Mixed within this epithelium are goblet cells, specialized endothelial cells
[8]. Goblet cells produce mucins, highly glycosylated glycoproteins, that allow them to form a mucosal layer atop a tissue
[9]. Ocular goblet cells secrete a mucin called MUC5AC, which is a known marker for their identification
[9]. This secretion is part of the body’s innate immune response and the failure to produce it may lead to an increased risk of infection
[8]. Goblet cells are also noted to secrete RELM-β, which is bactericidal, and zymogen granule protein 16 (ZG16), which aggregates bacteria and stops them from adhering to the body’s epithelium
[8].
Recent work has indicated that goblet cells are able to pass antigens to dendritic cells through what is called goblet cell-associated antigen passages (GAPs), which can lead to an inflammatory response
[10]. This has led to the conclusion that goblet cells play a significant role in immune tolerance of the ocular surface, and immune tolerance can be lost when goblet cells are absent
[10]. Other cells found in the bulbar conjunctiva are Langerhans cells, melanocytes, and lymphocytes
[6]. The bulbar portion of the conjunctiva relies on tight junctions, gap junctions, and desmosomes for selective permeability. This is all built on a basement membrane of type IV collagen which rests on the substantia propria, a loose connective tissue with high vascularization
[6]. This is in turn loosely connected to the underlying Tenon’s capsule
[6].
The conjunctival fornix is continuous with the skin while also connecting the bulbar and palpebral conjunctiva. It contains nonkeratinized stratified squamous epithelium
[6] in three layers: cylindrical, polyhedral, and cuboidal
[6]. Goblet cells, melanocytes, and dendritic cells are also interspersed
[6]. The substantia propria of this portion is thicker since it contains two parts: a superficial lymphoid layer, which contains connective tissue with lymphocytes, mast cells, plasma cells, and neutrophils, and a deeper fibrous layer that contains nerves, vessels, and the glands of Krause.
The palpebral conjunctiva lines the inner surfaces of the eyelids, extending from the mucocutaneous junction of the eyelid to the fornices
[6]. The palpebral conjunctiva is nonkeratinized stratified squamous epithelium and contains cuboidal epithelial cells and columnar epithelial cells. It also contains Langerhans and goblet cells
[6]. The palpebral conjunctiva contains regions: the marginal, tarsal, and orbital conjunctiva. The marginal conjunctiva contains the glands of Walfring and is where the transition from nonkeratinized stratified epithelium of the eyelid to cuboidal epithelium of the tarsal conjunctiva occurs
[6]. The tarsal conjunctiva also contains infolds containing goblet cells which are called the pseudo glands of Henle
[6]. The orbital conjunctiva extends all the way to the fornix and folds when the eye opens.
2.1.3. The Tear Film
The tear film of the eye, composed of water, mineral salts, antibodies, and lysozymes, is essential to ocular function and health
[11]. The tear film plays a role in clear vision, maintains epithelial cell health, and is part of the body’s innate immune response
[11]. It creates a smooth surface for refraction, supplying approximately two-thirds of the refraction of the eye
[11], and is responsible for eye lubrication, a very necessary component of comfort. A healthy tear film is approximately 6 μm thick and protects against potential irritants as well as flushing the eye when needed
[11]. Foreign bodies and other irritants will increase the production of tears as part of the body’s self-defense. Tear production is a result of a reflex loop that is driven by the nerves of the ocular surface, central nervous system, and glands of the ocular surface, sometimes collectively referred to as the lacrimal functional unit
[11].
There is a noteworthy chemical distinction between tears caused as part of an emotional response and those stimulated by an irritant. Protein-based hormones, prolactin, adrenocorticotropic hormone, and leucine encephalin, all compounds produced under stressful conditions, are more predominantly present in emotional tears as a mechanism to expel them from the body. The three major components of tears are made up in three distinct anatomical layers: oil, water, and mucus produced by the meibomian glands which line the edge of the eye, the lacrimal gland underneath the outer orbital rim bone, and the goblet cells in the conjunctiva, respectively
[12]. Tear film is spread evenly across the surface of the eye with every blink, and the motion then forces the tears into the puncta (drains) located in the corners of the upper and lower eyelids
[12]. Tear film travels from the puncta into the upper and lower canaliculi which empty into the lacrimal sac; from there, it is drained into the nasolacrimal duct that connects to the nasal passage
[12].
The tear film contains several different antimicrobials such as peroxidase, lactoferrin, lysozyme, and immunoglobulin A
[13]. The tear film also contains glucose, electrolytes, and growth factors for supporting the cornea, which is avascular. Overall, the composition of the tear film would be a dilute protein solution similar to serum, but with different concentrations of its various parts
[11]. There are two sections to the tear film: a lipid top layer that prevents evaporation and a mucin gel called the glycocalyx gel beneath that executes the many functions of the tear film. The lipid portion is secreted by the meibomian glands and has a low surface tension to allow the tear film to spread uniformly, with polar lipids preferring to locate themselves against the aqueous layer and nonpolar lipids moving toward the lipid–air interface
[11]. As mentioned earlier, the aqueous layer is similar in composition to serum, especially for electrolyte concentration. The main and accessory lacrimal glands, specifically the glands of Krause and Wolfring, produce the aqueous component constantly
[11]. The aqueous component of the tear film starts with a very low concentration of mucins near the lipid layer and sees an increase in concentration as it approaches the corneal epithelium. The gel is mostly hydrophobic glycoproteins that help the matrix firmly attach to the corneal epithelium, as well as increasing viscosity and lowering surface tension to help with keeping the hydrophobic ocular surface wet uniformly
[6][11]. Transmembrane mucins, which help with anchoring the matrix, are found in the corneal and conjunctival epithelium
[11]. The microvilli of the squamous epithelium of the cornea interact with the mucins in the tear film, helping to anchor and stabilize the tear film
[11]. There are cell-surface-associated mucins that form the glycocalyx, while secreted mucins are either soluble (closed to the tear film limit layer) or gel-forming (located near the conjunctival apical cells)
[6].
The meibomian glands are responsible for producing the oils that keep the aqueous portion of the precorneal tear film from evaporating
[5]. They are located in a portion of the eyelid called the tarsus, which is located behind the eyelashes. On average there are 25 meibomian glands in the upper eyelid and 20 in the lower eyelid
[5]. During the development, they differentiate from the pilosebaceous unit, same as eyelash follicles, so there are some conditions where this gland can be replaced by an eyelash
[5].
The glands of Krause are located in the conjunctival fornix. They are an accessory lacrimal gland, with 42 located in the superior fornix and 6–8 located in the inferior fornix. They, like the main lacrimal gland, produce the aqueous component of the tear film
[6]. The glands of Wolfring, also known as the glands of Ciaccio, are located within the palpebral conjunctiva, specifically above or within the tarsus
[6]. They are another minor accessory lacrimal gland and also produce tears
[6].
Tears act as a vehicle for the delivery and excretion of nutrients and metabolic products of the corneal epithelium and anterior stroma
[14]. The quality of vision is also a function of the stability of the tear film which keeps the surface of the eyes smooth and clear and serves as a protective barrier to infectious agents
[15]. The viscosity of tears is low (1–10 mPa-s) and is known to exhibit non-Newtonian properties, thus having a dependency on sheer rate
[14]. The reported flow rate for normal tear flow is approximately 1.2 µL/minute
[16], is driven by a pressure gradient, and is influenced by the rate of evaporation and production
[17]. A 2009 study examined the contribution of tangential flow to tear thinning and breakup between blinks and found that this flow is generally too slow and thus evaporation accounts for the bulk of tear thinning
[17]. Pressure gradients and gravity also give minor contributions to this event
[17]. A viscoelastic component of the lipid layer of tear fluid has been modeled to describe the upward movement of the fluid after a blink
[17]. Once the uneven lipid tension driving the movement becomes nearly uniform, the movement terminates
[17]. A Reynolds number has not been reported for tear flow, but a relatively low value is suggested by the assumed laminar flow used in models
[17].
The eye also must drain the fluid from its surface. The lacrimal drainage system passes liquid from the eye to the nose. Liquid enters the puncta, two drainage points located on the posterior of the eyelid margin, one on the upper and one on the lower lid
[5]. These look like small indents when an eyelid is observed in the mirror. These pass the liquid to the canaliculi, tubes that eventually fuse before meeting the lacrimal sac
[5]. The lacrimal sac can store some fluid and connects to the nasolacrimal duct which allows fluid to exit the nose by way of the inferior turbinate
[5].
2.2. Interface
A drug is typically delivered to the eye in the form of a free drug in an aqueous suspension administered as a liquid drop or ointment. Most surface drug delivery is noninvasive, is placed directly onto the surface, and diffuses directly into the eye. The main issue with these carrier systems is that ocular tissue is highly sensitive, and an incorrectly formulated drug or delivery mechanism will lead to ocular irritation, inflammation, and vision interference
[18]. The ocular surface has several natural barriers to drug absorption. Drainage abilities of the ocular surface clear many drugs before they can be absorbed, absorption by the conjunctiva is nonproductive, and the cornea is lipophilic which complicates the delivery of hydrophilic compounds
[19].
Precorneal fluid drainage is a main cause of low ocular drug absorption
[20]. After administration, approximately 80–95% of the initial dose volume is drained into the nasolacrimal duct which is meant to help maintain the precorneal fluid volume to about 7–10 µL
[21]. Along with excess fluid presence, addition of a fluid with pH varying from 7.4 (the pH of tear fluid) will result in excessive tear secretion and loss of drug
[20]. Tears will also dilute any hypertonic solutions they encounter, requiring the treatment solution to be isotonic with tears
[20].
The diffusion of a drug into the eye is controlled by the epithelium of the cornea. Due to the lipoidal nature of the epithelium, the treatment solution must exhibit intermediate solubility in the lipid layer to be effectively absorbed
[22]. The lamellar stroma is predominately aqueous, which requires the treatment solution to exhibit intermediate solubility in the aqueous layer and lipid layers for effective absorption
[23]. The presence of leaky tight junctions can allow for the passage of macromolecules across the corneal epithelium and is mediated by local osmotic gradients as well as the sodium pump
[24]. However, even if the applied drug can diffuse through the corneal epithelium, the treatment often fails to reach the retina and vitreous humor with sufficient concentration
[23][25][26][27].
The conjunctiva and sclera are considered minor pathways for drug delivery compared to the corneal route. Transport of hydrophilic solutes across the conjunctiva is limited due to tight junctions between epithelial cells
[28]. The sclera, consisting of mostly collagen, is more permeable than the cornea, but less so than the conjunctiva
[23]. Ocular drugs can be absorbed via the conjunctiva and delivered to the eye via the sclera, but this route is considered nonproductive due to the drainage loss through blood vessels in the conjunctiva
[23].
2.2.1. Topical Liquids and Solutions
Topical liquids are popular due to their relatively noninvasive mode of delivery
[18]. Their most common form is eyedrops
[18]. Through topical solutions, a drug is administered into the precorneal pocket; however, typically only 0–20% of the administered drug is retained, and the rest is lost to blinking
[18][25][29][30][31][32][33]. In addition, the potency, bioavailability, and clearance of the drug at the target ocular tissue are all factors that affect parameters such as required drug loading, release rate, and ocular retention times of drug delivery systems
[34]. Additionally, the material properties and size constraints of the eye limit drug-loading capacity
[34].
To treat conditions that affect the cornea and conjunctiva or tissues surrounding the anterior chamber (e.g., anterior segment diseases, inflammation, minor infections), it is typically sufficient to apply the drug directly to the ocular surface via eye drops, where the drug will mix with the lacrimal fluid. However, to be effective, the drug must remain in the tear film or become absorbed by the cornea or conjunctiva
[34]. If intraocular tissues such as the trabecular meshwork, iris, or ciliary body are the target of the drug, then it is necessary for the drug to permeate through the cornea and conjunctiva. Topical drug application is typically not effective for administering a drug to intraocular tissues, as effective concentrations of the drug do not reach the posterior segment. In most cases, topically applied drugs can permeate across the cornea but travel no deeper than the aqueous humor. Tight junctions in the corneal epithelium majorly restrict drug absorption
[35], but drugs diffuse freely through the corneal stroma and corneal endothelium
[36]. Once a drug reaches the aqueous humor, it can diffuse easily to the intraocular tissues. However, the distribution of drugs further into the vitreous and retina is limited by the physical lenticular barrier, aqueous humor turnover, and blood flow in the iris and ciliary body
[37].
Aqueous humor turnover and the blood flow in the iris and ciliary body are sufficient to eliminate small-molecule drugs, and aqueous humor turnover can clear large-molecule drugs
[38][39]. Hydrophilic small-molecule drugs can diffuse across the conjunctiva and sclera from the ocular surface to the iris and ciliary body without entering the aqueous humor. It is possible that large molecule drugs can also enter the iris and ciliary body via this route, as the openings in the conjunctival epithelium are larger than those in the cornea
[35][40][41].
Depending on molecular weight, hydrophobicity, size, etc., drugs can passively diffuse across the cornea. Various additives can be added to topically applied drugs to improve their contact time, permeation, and ocular bioavailability. These additives include viscosity enhancers, permeation enhancers, and cyclodextrins
[18]. Viscosity enhancers improve precorneal residence time and bioavailability
[18], which can help reduce drug loss due to blinking. Permeation enhancers slightly compromise corneal integrity to improve corneal uptake and drug bioavailability, but some studies have shown that they can cause local toxicity
[18]. Cyclodextrins can carry hydrophobic drugs in aqueous environments, which aids in delivering hydrophobic drugs to highly lipophilic biological membranes
[18]. The lipophilic membranes have a low affinity for the cyclodextrins themselves but a higher affinity for the hydrophobic drugs, which causes the cyclodextrins to remain in the aqueous solution when the drug is absorbed by the membrane
[18].
2.2.2. Emulsions and Microemulsions
Emulsions are colloidal systems with improved stability and drug bioavailability as compared to topical medications
[18]. There are two types of emulsion systems: oil in water (o/w) and water in oil (w/o)
[18][42][43][44]. For optical drugs, o/w systems are preferred, as they cause less irritation and are better tolerated than w/o systems
[18]. Emulsions are known to increase the bioavailability, permeation, and residence time of the drug they are delivering
[18][44].
Microemulsions are such systems between 5 and 200 nm and show significant thermodynamic stability, low surface tension, and enhanced drug retention time leading to greater absorption
[44]. Microemulsions are a particularly attractive option for ocular drug delivery due to their effectiveness at delivering poorly water-soluble drugs
[44][45][46][47][48][49][50][51] and their optical transparency
[52]. They have been effective in delivering drugs targeted to treat glaucoma, uveitis, keratitis, and bacterial and fungal infections of the eye
[44]. Unfortunately, the significant downside associated with the use of microemulsions is the large quantity of surfactant required to form stable microemulsions. A high concentration of surfactant on the surface of the eye could cause ocular toxicity. Depending on the particulars of a case, the use of a nonspontaneous preparation process in conjunction with coarse emulsions may be justified to reduce the risk of ocular toxicity. This issue can also be remedied by pursuing nonionic surfactants such as sugar ester surfactants and polysorbates such as Tween 60 and Tween 80
[44][53][54][55][56] which reduce both toxicity and ocular irritation
[44].
2.2.3. Suspensions and Nanosuspensions
Suspensions are used for the delivery of insoluble pharmaceuticals, generally by dispersing them in an aqueous solvent
[18]. Particle size is a substantial indicator of drug residence time and activity; small particles in the precorneal pocket replenish the drug absorbed by ocular tissue, while large particles are more easily retained in the precorneal pocket and slow drug dissolution
[18]. Suspensions require a “dissolution or release” of a drug prior to absorption
[57]. Release, ocular residence time, and bioavailability of a drug all vary based on the physicochemical properties of the suspension
[58]. In a rabbit model, Vooturi et al. investigated budesonide solutions at different viscosities and determined that an increase in viscosity significantly improved the ocular bioavailability to the aqueous humor
[57].
Nanosuspensions have similarly been shown to improve the bioavailability of hydrophobic drugs by improving solubility and residence time, with the only drawback being physical stability and the potential for drug sedimentation
[59]. Ali et al. demonstrated a 1.8-fold improvement in the bioavailability of hydrocortisone when prepared as a nanosuspension as compared to the commercially available solution
[60].
2.2.4. Ointments
Ointments can improve bioavailability and sustained release of ophthalmic drugs
[18]. An ointment is a mixture of semisolid and solid hydrocarbons that has a melting point at ocular temperature (34 °C)
[18]. Biocompatibility is the primary determinant of what hydrocarbon is selected for use in the ointment
[18]. Notably, ointments are a prevalent and effective way of delivering the broad-spectrum antibiotic vancomycin through minimally invasive means
[61]. As of 2019, at least three phase 3 clinical trials have been completed for vancomycin ointments that are intended to treat such conditions as bacterial conjunctivitis
[61][62] and have been previously used to treat blepharitis, conjunctivitis, and keratitis caused by MRSA and MRSE
[63].
2.2.5. Contact Lenses and Hydrogels
Contact lenses adhere to the tear film of the cornea using surface tension and were traditionally made of poly(methyl methacrylate) (PMMA); however, more recent lenses are made of hydrogels
[18][64]. Soft contact lenses are now a polymer blend (often of silicone and/or polyhydroxyethyl methacrylate (HEMA))
[64]. There are two types of contact lenses: hard and soft. Hard contacts are rigid but gas-permeable; soft contacts are flexible and made of high-water-content materials and are oxygen-permeable, an important feature for maintaining eye health. Softer lenses tend to fit the shape of the eye better due to their flexibility
[64].
Drugs delivered by contact lenses have longer residence times in the tear film and continuous drug delivery, leading to more drug entering the cornea
[18][30] and the advantage of greater than 50% bioavailability in comparison to traditional eye drop solutions
[29][65][66][67]. When designing contact lens drug delivery systems, there are several factors to consider, including lens transparency, oxygen permeability, glass transition temperature, wettability, and water content
[30]. Traditionally drugs have been loaded onto contact lenses by soaking them in solution; however, this is not an efficient means of loading and has only a short-term release
[18]. This has led to the creation of particle-laden contact lenses, where drugs are entrapped in vesicles dispersed in the contact lens material
[18]. For this method, implantation, nanoparticles, liposomes, microemulsion, and micelles are used
[29][65][68][69]. These and other novel polymer methods of delivering drugs have also been conceived, including molecular imprinting and use of vitamin E as barriers
[29][65][68][69]. However, since these vehicles are generally not covalently bound to the matrix, they can escape and cause irritation
[70]. These new methods rely on drug diffusion from the matrix, the degradation of the matrix, or the polymer responding to external stimuli, such as pH or temperature
[30][64]. Drug-eluting contact lenses have the unique concern that their optical or mechanical properties could change as the drug is lost; this has led some manufacturers to leave a clear central zone for the pupil
[70].
There are several areas of concern for contact lenses. The surface roughness can affect the ability of bacteria to adhere to them, so contact lenses with nanoparticles embedded may have a greater chance of bacterial adherence. Additionally, contact lenses may lose some of the drug they are loaded with during storage, which is something manufacturers need to be aware of when packaging and designing products. Finally, the contact lenses need to be both thin enough that they stay in the eye and transparent enough not to impede vision, which some modifications to lenses may not allow
[70].
In 2009, Ciolino et al. developed a solvent cast poly(lactic-co-glycolic acid) (PLGA) sandwich contact lens capable of releasing fluorescein and ciprofloxacin at a steady rate for a month with a minimal initial burst
[25]. This approach has since been used successfully to deliver econazole to inhibit fungal growth in vitro
[29][71], latanoprost
[29][72], and dexamethasone
[29][73]. Currently, there are several clinical trials either recruiting participants or under investigation involving eluting contact lenses of these same drugs (
clinicaltrials.gov accessed 13 May 2021). Most recent research efforts regarding drug-eluting contact lenses have looked towards developing bioresponsive and smart materials that deploy drugs based on received biosignals in vivo
[74].