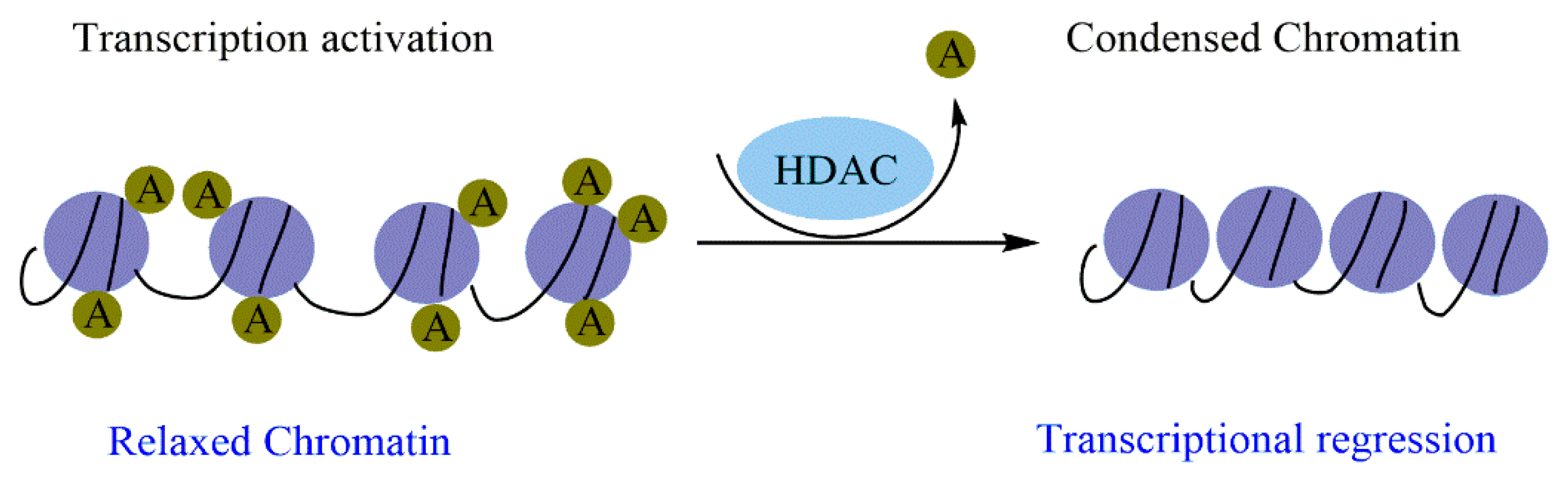
The implications of HDACs in cancer development were first reported in hematological malignancies by inappropriate involvement of HDAC-containing complexes
[73][74]. Until now, very rare mutations altering HDAC expression and activity have been recorded in tumors, while deregulation of their activity has been associated with abnormal gene expression and carcinogenesis. Many studies reported that HDAC1 is overexpressed in prostate, colon adenocarcinoma gastric, and breast carcinomas
[75][76][77][78], whereas HDAC2 is overexpressed in colorectal
[79], cervical
[80], and gastric cancers
[81]. Overexpression of HDAC1, HDAC2, and HDAC3 is linked to low survival in patients with gastric and ovarian cancers, while HDAC6 was highly expressed in breast cancer specimens
[82]. HDAC8 overexpression was reported in neuroblastoma, whereas low HDAC4 levels are reported in gastric cancers
[83][84].
Research findings indicated that knockdown of HDAC genes induced apoptosis and cell cycle arrest, particularly HDAC 1, 2, 3, and 6, in various cancers (colon, breast, lung, and acute promyelocytic leukemia (APL))
[76][84][85][86][87][88]. Knockdown of the HDAC4 gene inhibited cell proliferation and induced apoptosis
[89]. Class II HDACs are also involved in angiogenesis regulation, as the knockdown of HDAC6 and HDAC10 reduced angiogenesis-associated VEGFR1 and VEGFR2
[90]. Many reports showed up- or downregulation of SIRT genes in tumors. Along this line, SIRT1 was upregulated in lung cancer
[91], prostate cancer
[92], and leukemia
[45], and downregulated in colon tumors
[93]. Embryonic lethality, ascribed to reduced ability to repair DNA damage, was observed in mice lacking SIRT1
[94][95][96]. High levels of SIRT1 in AR-positive prostate cancer cell lines repress their multiplication. Indeed, SIRT1 can elicit senescence and avoid tumorigenesis
[97]. SIRT2 is frequently downregulated in human gliomas
[97][98][99].
3.1. HDAC in Different Cancer Stages
3.1.1. Cell Cycle Progression and Apoptosis
Several HDACs (from 1 to 6) are involved in tumor development, and their loss promotes cell proliferation dysregulation
[86][89][100][101][102]. HDAC1 reduces suppressors of the cell cycle mutually with Rb and by altering E2F1 activity
[103]. The inhibition of HDAC 1-2 induces cell cycle arrest
[104]. HDAC1 is also involved in G1/S and G2/M transitions. Another study also showed that HDAC1 knock-down contributes to G2/M phase arrest
[105]. Similarly, both HDAC3 and HDAC10 modulate the G2/M transition
[106][107]. High levels of Sp1 due to HDAC1/2/6 activities promote the division of cancer cells and G2/M progression
[108]. Knockdown of HDAC3 induced gathering of cells at the G2/M phase
[76], whereas in osteosarcoma cells, this effect causes siRNA-mediated HDAC1 depletion
[102].
Cell cycle interruption at the G2/M stage in renal cancer, following the inhibition of HDAC6 and HDAC3, has been ascribed to proteasomal alteration of Aurora B and A
[109]. Furthermore, SIRT1 can suppress the cell cycle through the blockage of p53-dependent pathways
[110]. HDAC11 negatively affects E2F7, E2F8, and cell cycle suppressors, leading to survival of tumor cells
[111]. Moreover, HDACs act as apoptosis regulators, as the interruption of this process is a critical factor for tumor progression and, therefore, is considered a hallmark of tumor progression. HDACs contribute to the extrinsic, as well as intrinsic, apoptotic pathways. Regarding the extrinsic apoptotic pathways, HDACs can obstruct TRAIL or TGF-b-mediated pathways, while pro- and antiapoptotic factors are altered in the intrinsic pathway
[112].
3.1.2. Differentiation
During differentiation, establishing a specific gene expression profile is harmonized by epigenetic modifications, e.g., histone acetylation. In this context, HDAC3 was recruited by RARPML
[113], while HDAC4 interacted with RAR-PLZF
[114] to repress differentiation-specific transcription. A close mechanism of retinoic acid signaling limitation in hematopoietic cells was recorded in AML1-ETO fusion proteins, which bind to HDAC1, 2, and 3
[115][116]. HDAC8 is a key regulator of cancer cell differentiation
[117], and HDAC8 overexpression is associated with neuroblastoma progression.
3.1.3. DNA Damage Response
HDACs contribute to DNA damage repair (DDR) responses via their key role in remodeling chromatin and regulating the acetylation patterns of proteins associated with DNA
[118]. The inhibition of HDAC blocks double-strand break (DSB) repair and radio-sensitizes cancerous cells. In this line, HDAC2 and HDAC1 bind to DNA damage regions, to deacetylate histones at H3K56 and H4K16 and promote non-homologous end-joining pathways, which accelerate DSB repair
[119]. Moreover, HDAC3 is involved in nucleotide excision repair (NER)
[120], whereas HDAC9 and HDAC10 contribute to homologous recombination
[121][122]. HDAC6, in association with DNA mismatch repair protein (MSH2), acts as an MSH2 inhibitor through deacetylation and ubiquitination
[123]. In addition, Sirtuins interact with numerous proteins regulating several DDR pathways
[124]. In tumor cells, SIRT1 restraints p53 acetylation, contributing to cell survival
[121][125]. SIRT6 phosphorylation is directly engaged in DNA damage sites to promote DSB repair
[126][127]. In leukemia-initiating cells, the inhibition of SIRT6 or HDAC8 engenders a DNA repair deficit in homologous recombination and the NHEJ pathway
[128].
3.1.4. Metastasis
The capacity to disperse and metastasize represents the deadliest signature of tumor cells. Numerous works have evidenced that HDACs regulate metastasis in various cancers. An important player in metastasis is the transition from adherent epithelial cells to motile mesenchymal cells capable of leaving the primary tumor site. In embryonic development, EMT is a crucial path for cell migration during gastrulation
[129]. In colorectal cancer, HDAC3 was engaged to Runx 2 promoter and hampered metastasis
[130]. Research findings showed that HDAC7 enhances EphA2 expression by downregulating miR-4465 expression, positively affecting tumor proliferation, spread, and invasion in nasopharyngeal cancer
[131]. Similarly, HDAC11 leads to the overexpression of RRM2, a gene involved in promigratory and metastatic phenotypes
[111]. In prostate cancer, ZEB1 and SIRT1 together bind to CDH1, promoting metastasis
[132], while SIRT1 elicited EMT through Fra-1 over-expression in colorectal cancer
[133].
3.1.5. Angiogenesis
Angiogenesis involves the creation and addition of new blood vessels and is pivotal for the development of tumors
[134][135]. The first steps of angiogenesis are elicited by hypoxia or a hypoxic microenvironment, while its advancement is mainly controlled by hypoxia-inducible factor 1a (HIF-1a). Overall, HDACs monitor the balance between pro- and anti-angiogenic proteins. In this context, HDAC inhibition exerts anti-angiogenic activity via inhibition of pro-angiogenic gene expression. Under hypoxia conditions, Class I HDACs, mRNA, and protein were overexpressed in vitro in primary and malignant cells
[136]. HDAC1 deacetylates HIF-1a, contributing to preventing HIF-1a loss. On the other hand, dysregulated levels of HDAC1 lead to high levels of HIF-1a and VEGF in tumors, which in turn enhances angiogenesis
[137]. HDAC4, 6, 10, and SIRTs display similar pathways
[112], whereas HDAC4, 5, and 6, acting as mediators of HIF-1 activity, require cofactors (HSP90 and p300)
[138]. On the contrary, it was shown that SIRT1 deacetylated HIF-1a, which reduces the interaction of HIF-1a with p300, which reduces HIF-1a activity. In endothelial cells, HDAC5 reduced the expression of pro-angiogenic genes (FGF2 and Slit2)
[139]. Additionally, HDAC5 represses cysteine-rich angiogenic inducer 61 (CYR-61), a well-known antifibrotic and pro-angiogenic mediator, inhibiting angiogenesis
[140]. HDAC6 enhances angiogenesis via deacetylation of cortactin, an actin-remodeling protein
[141].
3.1.6. Autophagy
Autophagy is a process that suppresses damaged subcellular fractions, helping to intercept the transformation of normal cells to cancerous ones
[142][143]. Published data showed that Class I HDACs mediate autophagic flux in mice
[36], whereas elimination of HDAC1 and HDAC2 impedes autophagic flux
[144]. On the other hand, HDAC4 and HDAC5 influence autophagic flux by acting as positive regulators of tumor cell development. HDAC6 promotes autophagy, based on its connection with microtubule proteins
[145][146]. In this regard, autophagy might be actively enhanced and play a compensatory role for HDAC6 under ubiquitin-proteasome system damage
[93][121]. At the same time, HDAC6 shows a significant role in ubiquitin-selective quality control autophagy, instead of starvation-induced autophagy
[147]. Similarly, Parkin-mediated mitochondrial ubiquitination could engage the autophagic actors, i.e., HDAC6 and p62
[148]. HDAC10 knock-down induces autophagosome/lysosome fusion blockade and restriction of autophagic flux, which sensitizes cells to chemotherapy
[149]. HDAC10 also deacetylates HSP70 protein members associated with autophagy-mediated cell longevity
[149]. In contrast, SIRT1 displays a dual role in autophagy
[150], where it is necessary to trigger starvation-induced autophagy
[100][151]. Moreover, SIRT1 deacetylates forkhead box O3 (FOXO3), leading to proteasomal degradation and, thus, contributing to the overexpression of numerous autophagic genes.
In embryonic stem cells (ESCs), SIRT1 affects the PI3K/Beclin 1 and mTOR pathways, affecting oxidative stress-induced autophagy
[152]. SIRT2 detaches from FOXO1 under stress conditions, which promotes hyperacetylated FOXO1, promoting the autophagic process
[153]. In contrast, deacetylation of lactate dehydrogenase B (LDHB) by SIRT5 intensifies its effect. Protons (H
+) generated by LDHB promote autophagy in tumor cells
[150][154]. Furthermore, SIRT5 is involved in ammonia-induced autophagy, via glutamine metabolism remodeling
[153]. SIRT6 promotes autophagy by hampering the transcriptional repressor Nkx3.2, resulting in the expression of GATA5
[155].