Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Cell Biology
MiR-424-5p has been widely identified as a tumor suppressor gene that functions in many types of human cancer. It is processed from the 5′ end arm of the miR-424 precursor, is located on human chromosome Xq26.3 and is clustered with miR-15/miR-16. MiR-424 is a member of the miR-16 family.
- miR-424-5p
- cancer
- tumor microenvironment
- tumorigenesis
- chemotherapy
1. Regulation of miR-424-5p Expression in Cancer
MiR-424-5p is a member of the miR-16 family. The miR-16 family can induce G1 arrest by simultaneously regulating multiple downstream effectors [18]. CCND1 is a cell-cycle-related gene that plays a key role in the G1/S transition. In addition, it is a potential target for tumor gene therapy. A reverse screening method proved that CCND1 was regulated by the miR-16 family. Moreover, one study showed that the miR-16 family regulates the expression of several other cell cycle genes, including cyclin D3 (CCND3), cyclin E1 (CCNE1) and cyclin-dependent kinase 6 (CDK6). All these data suggest that the miR-16 family induces G1 phase arrest by simultaneously regulating multiple downstream effectors [19]. Additionally, miR-16 and miR-424 can regulate the expression of CCND1 by targeting putative target sites [18]. At the same time, the ectopic expression of miR-424 was shown to lead to a significant decrease in the numbers of key cell cycle regulators, such as cell division cycle 25A (CDC25A), cyclin A2 (CCNA2) and CCNE1. The miR-424 binding site was found in the CCNA2 3′-UTR, and miR-424 targets CCNA2 directly by binding to the coding region of CCNA2, which can not only contribute to the differentiation of myoblast cells and cellular processes such as differentiation and epithelial-to-mesenchymal transition of cancer cells but also modulate the chemosensitivity of tumor cells toward anticancer drugs. Therefore, it can block the development of cancer [20]. Although various external stimuli affect miRNAs, the miR-16 family seems to be unique in its specific cell cycle-dependent regulation. The miR-16 family can regulate cell proliferation and/or apoptosis pathways in various tumor cells, participate in cell growth and inhibit cancer progression [19].
MiR-424-5p can be regulated by transcription factors (BFAR, bFGF, CCNE1J, MIEF2, E2F7) and regulatory proteins (WEE1, CARM1, DCLK1) in many cells. Moreover, the expression of miR-424-5p may also be affected by lncRNAs(long non-coding RNAs). In cancer cells, the abnormal regulation of these processes mentioned above leads to an imbalance in miR-424-5p, which leads to the occurrence and development of tumors.
2. MiR-424-5p as a Tumor Suppressor
MiR-424-5p can inhibit tumor proliferation and migration by targeting certain genes (WEE1, E2F7, KIF23, MIEF2, CARM1, Notch) or regulating protein expression. The following cancers are discussed to demonstrate some related miR-424-5p mechanisms.
2.1. Hepatocellular Carcinoma
MiR-424-5p has been fully studied in hepatocellular carcinoma (HCC). Hepatocellular carcinoma is a malignant tumor derived from hepatocytes and hepatobiliary cells and is a common malignant tumor in China. With the increase in alcohol intake and the spread of hepatitis virus, China has become one of the countries with the highest incidence of liver cancer [44]. In these studies, it was found that the expression of miR-424-5p in tissues and cells of patients with HCC was decreased, and as verified by dual-luciferase reporter gene assays, the proliferation, invasion and migration of HCC cells were inhibited by targeting the inhibition of the expression of YAPI [45], TRIM29 [46], E2F7 [47] or ICAT [48]. Zhu [49] also found an abnormal increase in the level of the autophagy-related protein ATG14 in HCC tissues and cell lines. Bioinformatics results showed that X-inactivation specific transcript (XIST) could negatively regulate ATG14 by binding multiple miRNAs, including miR-424-5p, to reduce the proliferation and migration of HCC cells. Therefore, miR-424-5p can reduce the malignancy of hepatocellular carcinoma cells in HCC.
Some studies [50] have also found that downregulated DLX6-AS1, by targeting miR-424-5p, suppresses the expression of WEE1, which has been shown [21] to be a nuclear kinase that regulates the G2-M transition, and thus inhibition of WEE1 may be a potential targeted therapy for cancer by inhibiting the proliferation of HCC cells.
Some clinical studies also demonstrated [48] that compared with nonmetastatic HCC patients, the expression of miR-424-5p in liver cancer tissue and the serum of metastatic HCC patients was significantly lower and that the expression of miR-424-5p was lower in patients with a higher pathological grade and a higher TNM stage.
In recent years, an increasing number of studies have investigated the signaling pathways related to miR-424-5p in HCC cells. Meta-analysis by some scholars [51] showed that PVT1 reduced the expression of miR-424-5p through the regulation of the p53 signaling pathway, thus affecting carcinogenesis and promoting the proliferation of HCC. Some studies have also shown that [22] lncRNA MYLK-AS1 upregulates E2F7 by targeting miR-424-5p directly to activate the VEGFR-2 signaling pathway, thus promoting angiogenesis and cell proliferation of HCC tumors in vivo and in vitro.
As mentioned above, lncRNAs can regulate other RNA transcripts by targeting specific miRNAs. LncRNA CDKN2B anti-sense RNA 1 (CDKN2B-AS1), which is an anti-sense RNA of cyclin-dependent kinase inhibitor 2B (CDKN2B), plays an important role in many diseases, including cancer. It was demonstrated in [52] that the expression of miR-424-5p in HCC decreased, while lncRNA CDKN2B showed the opposite expression. A dual-luciferase assay verified that the target gene of lncRNA CDKN2B-AS1 was miR-424-5p. The overexpression of miR-424-5p decreased cell viability, inhibited the cell migration and invasion capacities, and affected epithelial-mesenchymal transformation (EMT). Some studies [53] have also shown that miR-424-5p can be identified as the downstream target gene of lncRNA CASC9. LncRNA CASC9 promotes the proliferation, migration, invasion and apoptosis of HCC cells in vitro by negatively regulating miR-424-5p.
Some experimental results showed that miR-424-5p could reverse the recovery of malignant behavior in HCC, which further confirmed the anticancer function of miR-424-5p in HCC (Figure 2). This provides a novel and promising treatment strategy against liver cancer.
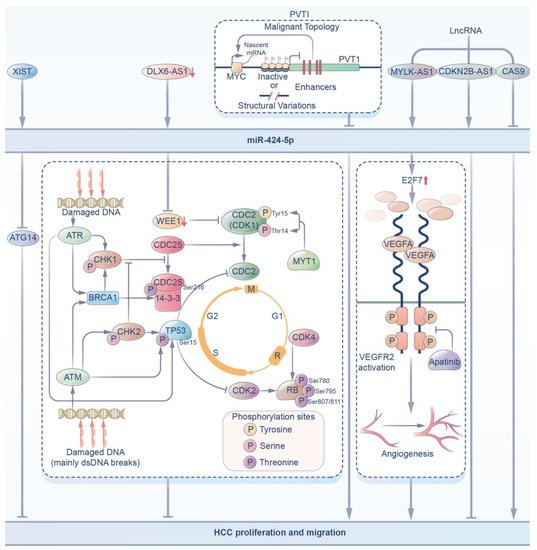
Figure 2. In HCC, miR-424-5p can be regulated by upstream pathways and regulate downstream pathways. (i) XIST can increase the expression of miR-424-5p, which inhibits ATG14, inhibiting HCC proliferation and migration. (ii) The decrease in DLX6-AS1 can target miR-424-5p to inhibit the expression of WEE1, which inhibits HCC proliferation and migration. (iii) PVT1 inhibits the expression of miR-424-5p by regulating the p53 signaling pathway to promote HCC proliferation. (iv) LncRNAs, including MYLK-AS1, CDKN2B-AS1, and CAS9, can affect the expression of miR-424-5p to regulate HCC progression.
Another type of liver cancer is intrahepatic cholangiocarcinoma (ICC) [54], which is a malignant tumor originating from intrahepatic bile duct epithelial cells, and its incidence has increased significantly in recent years. Only a few studies [23] have shown that the expression of miR-424-5p is downregulated in ICC and inhibits mTOR phosphorylation through targeted regulation of ARK5 in vitro, thus inhibiting the migration, invasion and epithelial-mesenchymal transition of ICC cells but having no effect on proliferation. This may be a new way to inhibit ICC metastasis.
In summary, miR-424-5p is expressed as a tumor suppressor in liver cancer, and the recovery of its expression may be a promising strategy for the treatment of liver cancer.
2.2. Ovarian Cancer
Ovarian cancer (OV) is one of the most common malignant tumors of the female reproductive organs. Its morbidity and mortality account for approximately 4% of all cancers in the world [55], second only to cervical cancer and uterine body cancer. Among these, epithelial ovarian cancer (EOC) has the highest mortality rate among all the types of gynecological tumors and poses a serious threat to the lives of women. OV has always been considered to be a hormone-dependent cancer, and therefore hormone receptors have become the primary research focus for targeted therapy for ovarian cancer [56]. Studies by Wang [42] and others have shown that the estrogen receptor (ESR1) transcriptionally regulates LINC00511 expression. Upregulated LINC00511 stimulates the proliferation of ovarian cancer cells by inhibiting apoptosis, while this long non-coding RNA inhibits the expression of a series of miRNAs including miR-424-5p and increases the expression of oncogenes. Therefore, miR-424-5p shows an active anticancer effect in OV. In addition, some studies have shown that miR-424-5p negatively regulates tumor-induced hypertrophy by directly targeting acyl-CoA synthetase long chain family member 4 (ACSL4) in OV cells, and subsequently reduced erastin- and RSL3-induced ferroptosis. This provides a new idea and method for OV therapy [24].
The metastasis of ovarian cancer is closely related to mortality, and therefore the biological characteristics of the tumors are also being actively explored. Liu et al. [57] showed that compared with normal tissues and cell lines, the level of miR-424-5p was significantly downregulated in EOC, which was negatively proportional to the TNM stage, tumor size and metastatic degree of EOC. The experimental results showed that miR-424-5p led to the arrest of the cell cycle in the G1 and S phases by targeting the CCNE1-mediated E2F1-pRb signaling pathway, which led to an antitumor effect on ovarian cancer. Similarly, Tong et al. [25] confirmed that the miR-424 cluster was significantly decreased in ovarian cancer due to hypermethylation of its promoter, in which miR-424-5p directly inhibited KIF23, while KIF23 promoted cell proliferation and migration in vitro. The knockdown of KIF23 affected the distribution of the cell cycle, the percentage of cells in G1 phase increased significantly, and the percentage of cells in S phase decreased significantly. It is suggested that the decrease in miR-424-5p can enhance the expression of KIF23 and inhibit the proliferation and migration of ovarian cancer cells. Recently, it has been shown [58] that the downregulation of miR-424-5p leads to the overexpression of MIEF2 (mitochondrial elongation factor 2) in OV tissues and cell lines, while the overexpression of MIEF2 significantly promotes the metabolic transition from oxidative phosphorylation to glycolysis in OV cells, and that the change in glucose metabolism characterized by increased glycolysis (also known as the Warburg effect) has been recognized as one of the markers of cancer [59] This leads to the occurrence, development and metastasis of tumors in OC.
Similarly, Cha et al. [60] showed that miR-424-5p is also a tumor suppressor in serous ovarian cancer. The expression of miR-424-5p in ovarian cancer was significantly lower than that in the normal group, and the decrease in miR-424-5p expression was significantly related to distant metastasis. Therefore, it is suggested that the decreased expression of miR-424-5p in ovarian cancer may be a recognized biomarker of distant metastasis of ovarian cancer.
From this series of studies, it can be suggested that miR-424-5p can target a variety of oncogene mRNAs and play a tumor inhibitory role in OV (Figure 3).
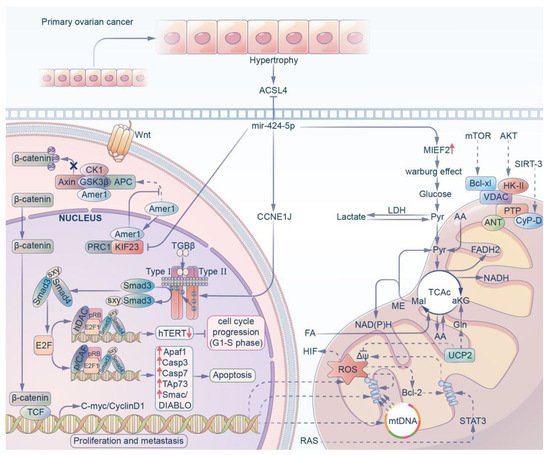
Figure 3. MiR-424-5p can regulate ovarian cancer progression in 4 different ways. (i) MiR-424-5p can inhibit ACSL4 to prevent hypertrophy of primary ovarian cancer. (ii) MiR-424-5p decreases the expression of KIF23 to inhibit the proliferation and migration of ovarian cancer. (iii) MiR-424-5p targets CCNE1J directly to activate the E2F1-pRb signaling pathway, which results in an antitumor effect. (iv) MiR-424-5p increases the expression of MIEF2 to facilitate the Warburg effect.
2.3. Cervical Cancer
Cervical cancer is one of the most aggressive cancers in women, with a high recurrence and mortality [61]. Previous studies have shown that the expression of miR-424 in cervical cancer tissue is decreased, which inhibits the proliferation and growth of cancer cells [62,63]. MiR-424-5p is a branch of the miR-424 family, and thus it has been studied in great detail. Dong [64] showed that lncRNA nuclear RNA host gene 12 (SNHG12) was abnormally elevated in human cervical cancer tissue, while silencing SNHG12 inhibited the proliferation of cervical cancer cells and tumor growth in a nude mouse model. By predicting that SNHG12 has a presumptive binding site related to the seed sequence of miR-424-5p, the experimental verification showed that the tumor-promoting effect of SNHG12 was through acting as a molecular sponge of miR-424-5p, thus negatively regulating the expression of miR-424-5p in cervical cancer to promote the proliferation and invasion of cervical cancer cells. Similarly, Zhou [26] demonstrated that the expression of miR-424-5p was significantly decreased in cervical cancer tissues and cells, while a dual-luciferase assay confirmed that KDM5B was the direct target gene of miR-424-5p. MiR-424-5p inhibited cell proliferation and migration and promoted apoptosis in cervical cancer cell lines by targeting the KDM5B-Notch pathway. The cervical squamous cell carcinoma mentioned above, which is a type of cervical cancer, demonstrated that miR-424-5p had anticancer effects. Therefore, miR-424-5p plays an overall role in inhibiting the occurrence and development of cervical cancer, which provides a new idea and scheme for clinical treatment in the future.
2.4. Neurological Malignancies
Glioma is one of the most common primary tumors of the nervous system, accounting for approximately 40–50% of intracranial tumors. Although great progress has been made in treatment in recent years, the prognosis of gliomas is poor due to the special properties of gliomas, such as invasive growth, recurrence, radiotherapy and chemotherapy resistance and other factors [65]. Therefore, gene targeting research provides a new path for treatment. It was concluded that bifunctional apoptosis regulator (BFAR) is a direct target of miR-424-5p. Cheng [27] showed that the content of miR-424-5p was decreased significantly in glioma tissues. As predicted by TargetScan and confirmed by bifunctional luciferase reporter gene analysis, BFAR is expressed negatively proportionally to miR-424-5p. MiR-424-5p could inhibit the progression of gliomas by decreasing Ki-67 expression, thus inhibiting invasion and proliferation.
Similarly, high levels of ALK protein in neuroblastoma (NB) are associated with metastatic NB cases and poor prognosis, while experimental results [66] have shown that miR-424-5p can directly target ALK receptors or indirectly regulate ALK expression in NB cells, resulting in a significant decrease in ALK protein and inhibition of cell viability in ALK-positive NB cell lines.
Zhou et al. [67] found a similar situation in pituitary neuroendocrine tumors (PitNETs). The specific transcriptional gene product of lncRNA X inactivation upregulates the expression of basic fibroblast growth factor (bFGF) by competitively binding to miR-424-5p to inhibit the proliferation, migration and invasion of invasive PitNET cells and promote cell cycle arrest and apoptosis of invasive PitNET cells.
Therefore, miR-424-5p may be a favorable supplement for combined therapy for the treatment of nerve cell malignant tumors.
2.5. Breast Cancer
Breast cancer (BC) is one of the most common cancers and therefore its pathogenesis and treatment have been the focus of much research. In recent years, there have been an increasing number of studies on targeted gene therapy. Experiments have proven that miR-424-5p is closely related to the occurrence and development of BC. Some experiments have shown that the overexpression of miR-424-5p in BC can inhibit cell proliferation and migration. A year later, Narges et al. [28] also confirmed the antitumor activity of miR-424-5p in BC cells and proposed the corresponding mechanism, which is that miR-424-5 can target PD-L1 and regulate the expression of the PTEN/PI3K/AKT/mTOR signaling pathway to inhibit cell proliferation and lock the cell cycle in the G2 phase. Another way that miR-424-5p inhibits tumor cell proliferation and arrests cells in G2/M cell phase is to activate the Hippo pathway and the extracellular signal-regulated kinase pathway by suppressing CDK1 mRNA in human breast cancer [68]. Similarly, brain-derived neurotrophic factor (BDNF) was proven to be the direct target protein of miR-424-5p in BC cells, and LINC00922 was shown to regulate the expression of BDNF by sponging miR-425-5p. LINC00922 is obviously overexpressed in breast cancer tissues and cell lines, and therefore increasing the expression of the miR-424-5p/BDNF axis can reduce the expression of LINC00922 and weaken its effects on promoting the proliferation, apoptosis, migration and invasion of breast cancer cells [69].
It has been demonstrated that natural-based molecules such as resveratrol can regulate the level of miRNAs. Resveratrol is a multi-role natural compound which has antitumor and anti-inflammatory roles. Besides this, it can also modulate miRNA expression. In BC cells, resveratrol regulates cancer cell growth by promoting the expression of miR-424-5p and inhibiting HNRNPA1. Moreover, HNRNPA1, which is related with tumor progression, is modulated by miR-424-5p [70].
For another subtype of BC, basal-like breast cancer [71], the expression of miR-424-5p is decreased in its tissues and cell lines, and the oncogene bicorticoid kinase 1 (DCLK1) is directly targeted to regulate tumor proliferation, invasion and migration. Patients with a low expression of miR-424-5p had higher clinical stages, larger tumor sizes and worse histological grades. In summary, miR-424-5p is a tumor suppressive miRNA in breast cancer that has therapeutic potential to enhance tumor immunity and inhibit the proliferation of tumor cells in BC.
2.6. Non-Small-Cell Lung Cancer
Non-small-cell lung cancer (NSCLC), as the most serious type of lung cancer with a high incidence, urgently needs new treatment strategies. Some studies [29] have shown that, in NSCLC tissues, miR-424-5p is poorly expressed, while lncRNA PVT1 and arginine methyltransferase 1 (CARM1) were highly increased. It is known that the combination of miRNAs and lncRNAs [72] can lead to the degradation of lncRNAs and affect the occurrence and development of tumors. Therefore, lncRNA PVT1 and CARM1 can bind to miR-424-5p. Overexpression of miR-424-5p inhibits the expression of CARM1 and increases the factors related to tumor progression and apoptosis, thus inhibiting tumor cell proliferation, migration and invasion and even improving the radiosensitivity of NSCLC. Similarly, YI [73] also showed that LINC00641 suppressed cancer in NSCLC by increasing the content of miR-424-5p. MiRNA and LncRNA are both key gene expression regulatory molecules [74], which play an important role in the occurrence and development of tumors. They can regulate each other with multiple sites and targets and interact and affect the performance of their respective functions. Therefore, in recent years, research on miR-424-5p has been increasingly related to lncRNAs. It is necessary to know that the disruption of the regulatory balance leads to tumorigenesis. Perhaps the key to treatment is to restore the balance of molecular regulation.
2.7. Osteosarcoma
Osteosarcoma is a malignant tumor that often occurs in adolescents and grows around the knee joint. It is the most common form of bone cancer [75]. Selvaraj [30] determined the function of melatonin in the osteosarcoma microenvironment. The experimental results confirmed that melatonin can inhibit tumor angiogenesis through the miR-424-5p/vascular endothelial growth factor A (VEGFA) axis, regulate the proliferation and migration of surrounding endothelial cells, the vascular morphology and the angiogenic growth factors and play a key role in tumor inhibition. Therefore, miR-424-5p can inhibit the proliferation, invasion and migration of cancer cells in osteosarcoma and provide new ideas for creating more effective treatments.
2.8. Nasopharyngeal Carcinoma
Nasopharyngeal carcinoma (NPC) is a malignant tumor originating from the mucosal epithelium of the nasopharynx. It is prevalent in southern China and Southeast Asia [76]. Finding molecular targets related to the malignant biological behavior of NPC is very important for improving the clinical treatment of NPC. Zhao collected 26 normal tissues and 40 NPC patient tissues [31]. It was found that the expression of miR-424-5p in NPC tissues was downregulated and negatively proportional to lymph node metastasis and clinical staging. It was also confirmed that AKT3 is the direct target of miR-424-5p and that miR-424-5p inhibits the proliferation, migration and invasion of NPC cells by reducing the expression of AKT3. The downregulation of miR-424-5p in NPC is closely related to the occurrence and development of NPC.
3. MiR-424-5p Acts as an Oncogene
MiR-424-5p can also improve cancer progression. In some specific cancers, miR-424-5p can bind to the target and promote tumor cell proliferation and migration. The mechanisms of miR-424-5p are briefly discussed.
3.1. Pancreatic Cancer
Pancreatic cancer is an invasive malignant tumor with high mortality. Because of its aggressive biological characteristics, the current treatment methods cannot greatly increase the prognosis of patients. Even surgical resection cannot significantly improve the survival rate of patients [77]. Therefore, it is necessary to explore unique molecular targets and biotherapy for pancreatic cancer. Wu et al. [32] showed that the expression of miR-424-5p was upregulated in pancreatic cancer. Dual-luciferase reporter gene detection showed that cytokine-induced signal transduction 6 (SOCS6) was the direct target of miR-424-5p. MiR-424-5p is significantly upregulated in pancreatic cancer and regulates the ERK1/2 signaling pathway through the negative regulation of SOCS6, which leads to the proliferation, migration and invasion of pancreatic cancer cells. Therefore, miR-424-5p may play a beneficial role in the diagnosis and treatment of pancreatic cancer.
3.2. Thyroid Cancer
Liu et al. [33] evaluated the expression of miR-424-5p in thyroid carcinoma and analyzed the miRNA dataset of The Cancer Genome Atlas (TCGA). The results showed that the expression of miR-424-5p in thyroid carcinoma was significantly higher than that in normal tissues. It was verified that the upregulation of miR-424-5p suppressed several downstream genes of the Hippo pathway in thyroid cancer cells. Dual-luciferase detection showed that WWC1, SAV1 and LAST2 were direct targets of miR-424-5p in thyroid cancer cells and were negatively correlated. Therefore, miR-424-5p suppresses the activity of Hippo signal transduction by targeting WWC1, SAV1 and LAST2, thus promoting lung metastasis and the anoikis resistance of thyroid cancer. Anoikis resistance refers to the capacity of tumor cells in suspension circumstances. Therefore, it represents the metastasis levels of cancer cells. The overexpression of miR-424-5p inhibits the activity of caspase-3 or -9 to promote anoikis resistance in thyroid cancer cells. This finding deepens our understanding of the molecular mechanisms underlying lung metastasis of thyroid cancer, which will provide new insights into the development of treatment strategies against lung metastases in thyroid cancer.
In addition, through bioinformatics analysis in the targeted regulatory network of miRNA–mRNA, it was shown that hsa-miR-424-5p may have the potential to synchronously regulate two central genes to reverse the process of papillary thyroid carcinoma. This may provide a new strategy for the treatment of thyroid cancer [78].
3.3. Gastric Cancer
Gastric cancer (GC) is one of the most common digestive tract malignant tumors. A large number of studies have shown that miRNAs play a key role in the development of GC by inhibiting the expression of target genes [79]. Studies by Jing [80] and others have shown that the overexpression of miR-424 promoted the proliferation and invasion of GC cells by targeting the LATS1 gene. Sequencing analysis showed that miR-424-5p was negatively and strongly correlated with the expression of LATS1, and therefore miR-424-5p could promote the proliferation of gastric cancer cells through LATS1. Similarly, Song et al. [34] examined the effect of miR-424-5p on the tumor growth of GC cells. The results showed that the overexpression of miR-424-5p promoted the proliferation of gastric cancer cells, while the knockdown of miR-424-5p could induce cell cycle arrest at the G0/G1 phase. Smad3 is known to be the direct target of miR-424-5p. MiR-424-5p promotes the proliferation of gastric cancer cells by targeting Smad3 through the TGF-β signaling pathway. Reducing the expression of miR-424-5p may be a new treatment that can be tried.
This entry is adapted from the peer-reviewed paper 10.3390/ijms23074037
This entry is offline, you can click here to edit this entry!
