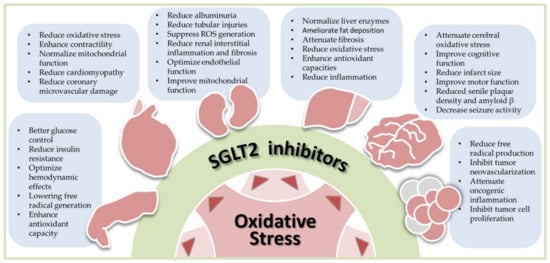
Insulin resistance is a critical feature in the pathogenesis of T2DM, which also aggravates oxidative stress; conversely, oxidative stress exacerbates insulin resistance, accelerating the progression of prediabetes and T2DM [
49]. Emerging investigations have indicated that SGLT2 inhibitors might influence insulin signal transduction, improve peripheral insulin sensitivity, and thus alleviate insulin resistance [
63]. Combined with the antioxidant mechanisms mentioned above, SGLT2 inhibitors could disrupt the vicious cycle between insulin resistance and oxidative stress and are therefore an excellent therapeutic option.
The benefits of SGLT2 inhibitors on insulin resistance originate from multiple mechanisms. In patients with diabetes, Goto et al. evaluated the influence of empagliflozin treatment on insulin sensitivity of skeletal muscles; following a one-week regimen, noticeable improvement in insulin sensitivity was detected, which was attributed to a rapid correction of glucotoxicity [
64]. Additional experimental and clinical studies have supported the benefits of SGLT2 inhibitors on β-cell function, insulin signaling, and insulin sensitivity via amelioration of glucotoxicity [
65,
66]. The effects of SGLT2 inhibitors on weight control and visceral fat lowering, attributed to glycosuria-related caloric deposition, are also crucial for insulin sensitivity. Studies in Japanese and Indian diabetic populations have demonstrated that SGLT2 inhibitors contribute to a reduction in body weight and visceral fat, improving insulin sensitivity via alleviation of lipotoxicity [
67,
68]. In animal models, SGLT2 inhibitors induced white adipose tissue browning and improved fat utilization via an M2 macrophage polarization-dependent mechanism, thereby increasing insulin sensitivity [
69]. To maintain better glucose homeostasis and enhance insulin sensitivity, preservation of β-cell function is imperative, and SGLT2 inhibitors reportedly play vital roles in terms of this aspect. Robust clinical evidence [
70,
71] on the β-cell protection afforded by SGLT2 inhibitors has been documented, with several relevant mechanisms proposed, including deceleration of β cell death and regeneration of pancreatic islet cells via amelioration of glucotoxicity, lipotoxicity, inflammation, fibrosis, and oxidative damage [
63,
72]. Furthermore, Wei et al. reported that SGLT2 inhibitors induced β-cell self-replication, α-to-β cell conversion, and duct-derived β cell neogenesis in an animal model, partially mediated by the additional promotion of glucagon-like peptide-1 (GLP-1) secretion [
73]. Finally, the attenuative effects of SGLT2 inhibitors on oxidative stress and inflammatory responses are also crucial for restoring insulin sensitivity, as discussed above.
In conclusion, SGLT2 inhibitors can decrease insulin resistance via multiple mechanisms, including glucotoxicity correction, caloric disposition and lipotoxicity modulation, β cell preservation, and attenuation of oxidative stress and inflammation. Collectively, the antioxidant properties and insulin-sensitizing effects of SGLT2 inhibitors could disrupt the interplay between oxidative stress and insulin resistance in T2DM and serve as favorable therapeutic options.