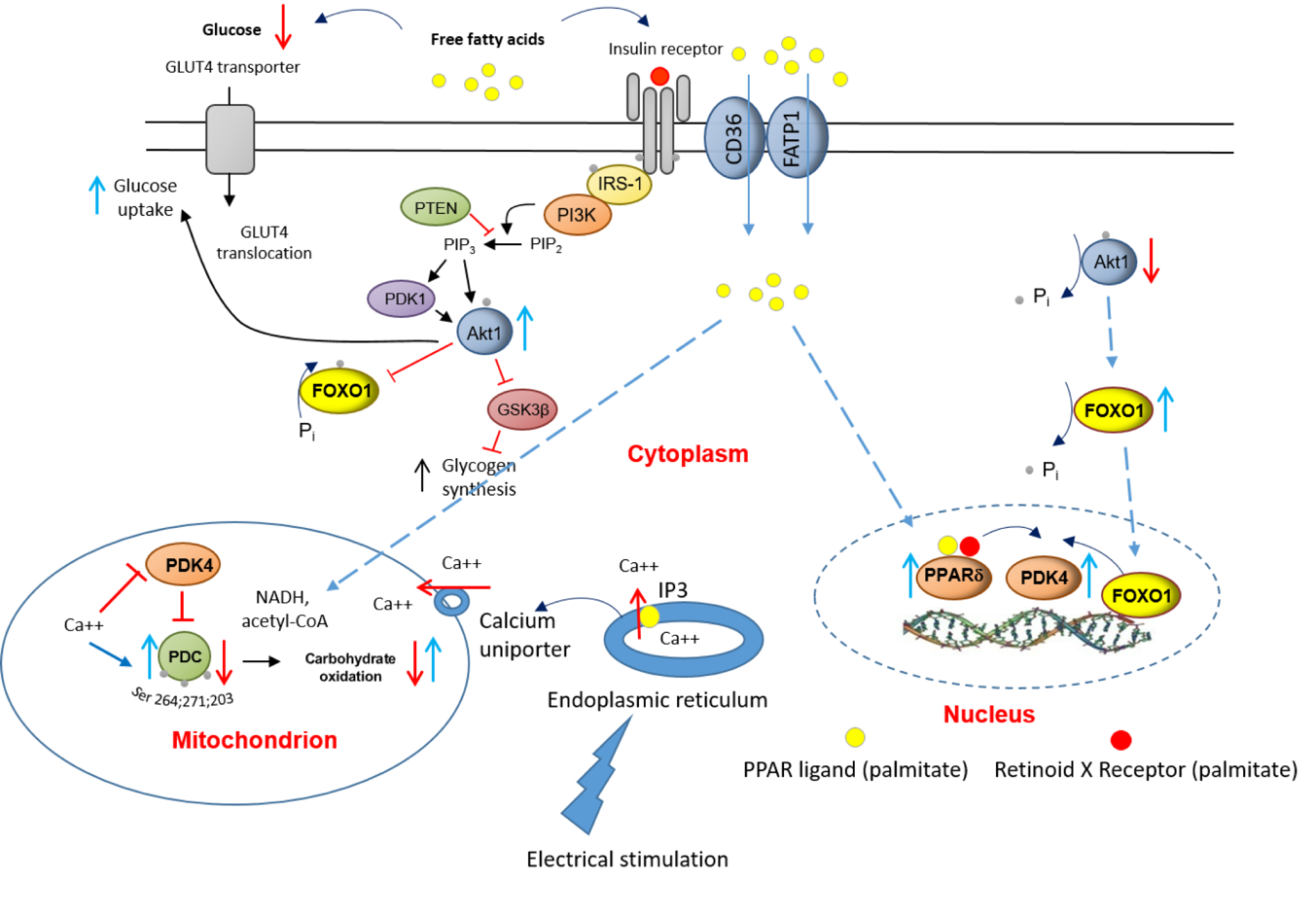
The scheme points to potentially important roles for PPAR delta and FOXO1 in the palmitate-induced increase in myotube PDK4 protein expression and linked reductions in cellular glucose uptake, PDC activity and flux, and rates of CHO derived oxidative ATP generation. Activation of PPAR delta with natural (free fatty acids) or synthetic ligands have been associated with increases in muscle PDK4 mRNA and protein expression, which would thereby inhibit PDC activity. In the case of FOXO1, dephosphorylation (activation) results in its translocation from the cytosol to the nucleus, where it can bind directly to the promoter region of the PDK4 gene. Moreover, FOXO1 dephosphorylation appears to be coupled via a signalling cascade to changes in circulatory FFA and/or insulin availability, thereby tying muscle PDK4 gene transcription (amongst others) to substrate availability. Furthermore, the finding that 16 hrs of EPS calcium release rescued this palmitate-induced dysregulation of cellular CHO metabolism via modulation of the same molecular and cellular events, alludes to the mechanisms by which chronic physical activity will protect skeletal muscle against lipid-induced muscle metabolic inflexibility and IR.
- fuel selection
- PPAR delta
- FOXO1
- PDK4
- PDC activity
Please note: Below is an entry draft based on your paper, which is wrirren tightly around the entry title. Since it may not be very comprehensive, we kindly invite you to modify it (both title and content can be replaced) according to your extensive expertise. We believe this entry would be beneficial to highlight your work.
Abstract
The mechanisms behind the reduction in muscle pyruvate dehydrogenase complex (PDC)-controlled carbohydrate (CHO) oxidation during chronic high-fat dietary intake are poorly understood, as is the basis of CHO oxidation restoration during muscle contraction. C2C12 myotubes were treated with (300 μM) palmitate or without (control) for 16 h in the presence and absence of electrical pulse stimulation (EPS, 11.5 V, 1 Hz, 2 ms). Compared to control, palmitate reduced cell glucose uptake (p < 0.05), PDC activity (p < 0.01), acetylcarnitine accumulation (p < 0.05) and glucose-derived mitochondrial ATP production (p < 0.01) and increased pyruvate dehydrogenase kinase isoform 4 (PDK4) (p < 0.01), peroxisome proliferator-activated receptor alpha (PPARα) (p < 0.01) and peroxisome proliferator-activated receptor delta (PPARδ) (p < 0.01) proteins, and reduced the whole-cell p-FOXO1/t-FOXO1 (Forkhead Box O1) ratio (p < 0.01). EPS rescued palmitate-induced inhibition of CHO oxidation, reflected by increased glucose uptake (p < 0.01), PDC activity (p < 0.01) and glucose-derived mitochondrial ATP production (p < 0.01) compared to palmitate alone. EPS was also associated with less PDK4 (p < 0.01) and PPARδ (p < 0.01) proteins, and lower nuclear p-FOXO1/t-FOXO1 ratio normalised to the cytoplasmic ratio, but with no changes in PPARα protein. Collectively, these data suggest PPARδ, and FOXO1 transcription factors increased PDK4 protein in the presence of palmitate, which limited PDC activity and flux, and blunted CHO oxidation and glucose uptake. Conversely, EPS rescued these metabolic events by modulating the same transcription factors.
Introduction
A schematic illustration of the underlying mechanism by which palmitate inhibits the carbohydrate (CHO)/glucose-derived pyruvate mitochondrial ATP production through activation of PPARδ- and FOXO1-mediated upregulation of PDK4 protein. Blue upright arrow denotes upregulation and red downward arrow denotes downregulation. CD36, fatty acid translocase; FATP1 insulin-sensitive fatty acid transporter; PDK1, 3-phosphoinositide-dependent kinase-1; GLUT4, glucose transporter isoform 4; IGF, insulin-like growth factor; FFA, free fatty acid; Akt1, serine/threonine-protein kinase 1; PTEN, phosphatase and tensin homolog; PI3K, phosphoinositol 3-kinase; GSK3β, glycogen synthase kinase; IRS-1, insulin receptor substrate 1.
This entry is adapted from the peer-reviewed paper 10.3390/ijms21165942