The fire extinguishing efficiency of C6F12O has been fully investigated in both a laboratory burner scale and full-scale fire extinguishing experiment, the results of which show that the minimum extinguishing volume concentration of C6F12O is lower than HFCs, but the mass concentration is much higher. Although C6F12O has shown satisfactory fire extinguishing performance in various fire protection scenarios, fire enhancement phenomenon and the large production of HF have been observed during fire extinguishment. Furthermore, the fire extinguishing mechanism of C6F12O has been discussed. The flame suppression effect of C6F12O, combustion enhancement phenomenon and the influence of water in the reaction zone have been revealed.
- C6F12O
- Novec 1230
- Fire Extinguishing Efficiency
- Fire extinguishing mechanism
- combustion enhancement
- Influence of H2O
1. Extinguishing Efficiency
1.1. Laboratory Scale Experiments
| Researcher | Fuel | Test Result of MEC | Note |
|---|---|---|---|
| Carnazza et al. [55] | n-heptane, alcohol and other liquid fuels | n-heptane 4.5%, alcohol 5.6% | |
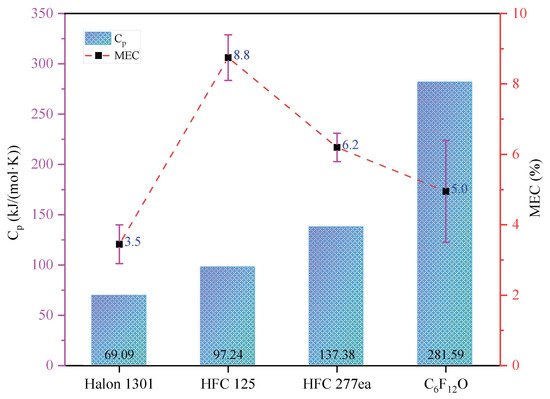
| Andersson et al. [24,56] | propane | 6.4% | lower than HFC 125 and HFC 227ea under the same experimental conditions, higher than Halon 1301 |
| Rivers et al. [14] | propane | 3.5% | lower than Halon 1301 and Halon 1211 under the same experimental conditions but the required mass for the same fire extinguishing efficiency is relatively high |
| Takahashi et al. [57] | propane | 4.17% | the calculated MEC is 4.12% |
| Li [54] | n-heptane | 4.5–5% | under different gasification heating temperature, air temperature, heating coil and environment temperature |
1.2. Full-Scale Experiment
| Researcher | Aim | Fire Scene | Result |
|---|---|---|---|
| Hodges et al. [69] | Evaluate the fire extinguishing efficiency of C6F12O and the generation amount of acid gas in specific scenarios | Military vehicle, 7.36 m2 chamber | The fire extinguishing efficiency of C6F12O is similar to that of halon and its substitutes, which can extinguish 7.36 m2 fire within 200 ms. However, the mass of fire extinguishing agent and the amount of acid gas in products cannot meet the application standard. |
| Bengtson et al. [70] | Fire extinguishing efficiency and re-burning of C6F12O in polymer fire ignited by different electric power | Polymer fire ignited by 192W electric power | The fire extinguishing concentration is less than Halon 1301 and higher than the n-heptane test result given by NFPA 2001. When the fire suppressant concentration is higher than the test value of cup burner, the fire can be prevented from re-ignition. |
| Kim et al. [71] | The fire extinguishing efficiency and acid gas generation of C6F12O | 3 kW cable fire, small oil pool and wood stack fire under the design concentration (6.5%) in 58 m3 space | It takes a long time for the extinguishing agent to reach the extinguishing concentration in the confined space. The cable fire is put out 72 s after combustion, while it is put out in 30 s in the open and ventilated environment. The wood stack and oil pool fire can be put out in 10 s. A large amount of acid gas is produced in a large flame. |
| Liu et al. [72] | Fire extinguishing efficiency of C6F12O for lithium batteries | 38 Ah prismatic ternary (Li(Ni1/3Co1/3Mn1/3)O2/graphite))battery with the voltage of 4.2 V in 47.5 * 21.5 * 16 cm3 module box | With the increase in agent concentration, the fire extinguishing effect first decreases and then increases. No obvious cooling effect of C6F12O was found in the experiment |
| FAA et al. [73] | Feasibility of fire extinguishing application of C6F12O in aviation application | Jet A fuel in the inclined-plane fire tests,16- and 30-ft pan tests and the simulated engine nacelle fire tests | It is similar to halotron I (CF3CHCl2/Ar/CF4 mixture), but the volume and mass of C6F12O are larger than halotron I |
2. Fire Extinguishing Mechanism
2.1. Physical Mechanism
2.2. Chemical Mechanism
Chemical Extinguishing Process of C6F12O
Influence of H Content in Reaction Environment on Fire Extinguishing Process
Flame Enhancement Mechanism during the Fire Extinguishment
This entry is adapted from the peer-reviewed paper 10.3390/fire5020050