In recent decades, Raman spectroscopy (RS) has been used in several studies on animal cells. The method is popular among biophysicists and life science researchers. RS allows for the study of living cells in their natural conditions without any damage. RS is a well-known approach used in many of biomedical studies. Since biomolecules are involved, the main obstacle to the use of such methods in life science research is the low signal of Raman scattering. There are a number of modifications of RS that allow Raman scattering to be improved. There are existing approaches to detect Raman signals not only on the surface of human skin, but also inside the vasculature and various organs of patients.
- Raman spectroscopy (RS)
- carotenoids
- Microalgae
- Lipids
- Cancer cells
1. The Principles of the Method of Raman Spectroscopy
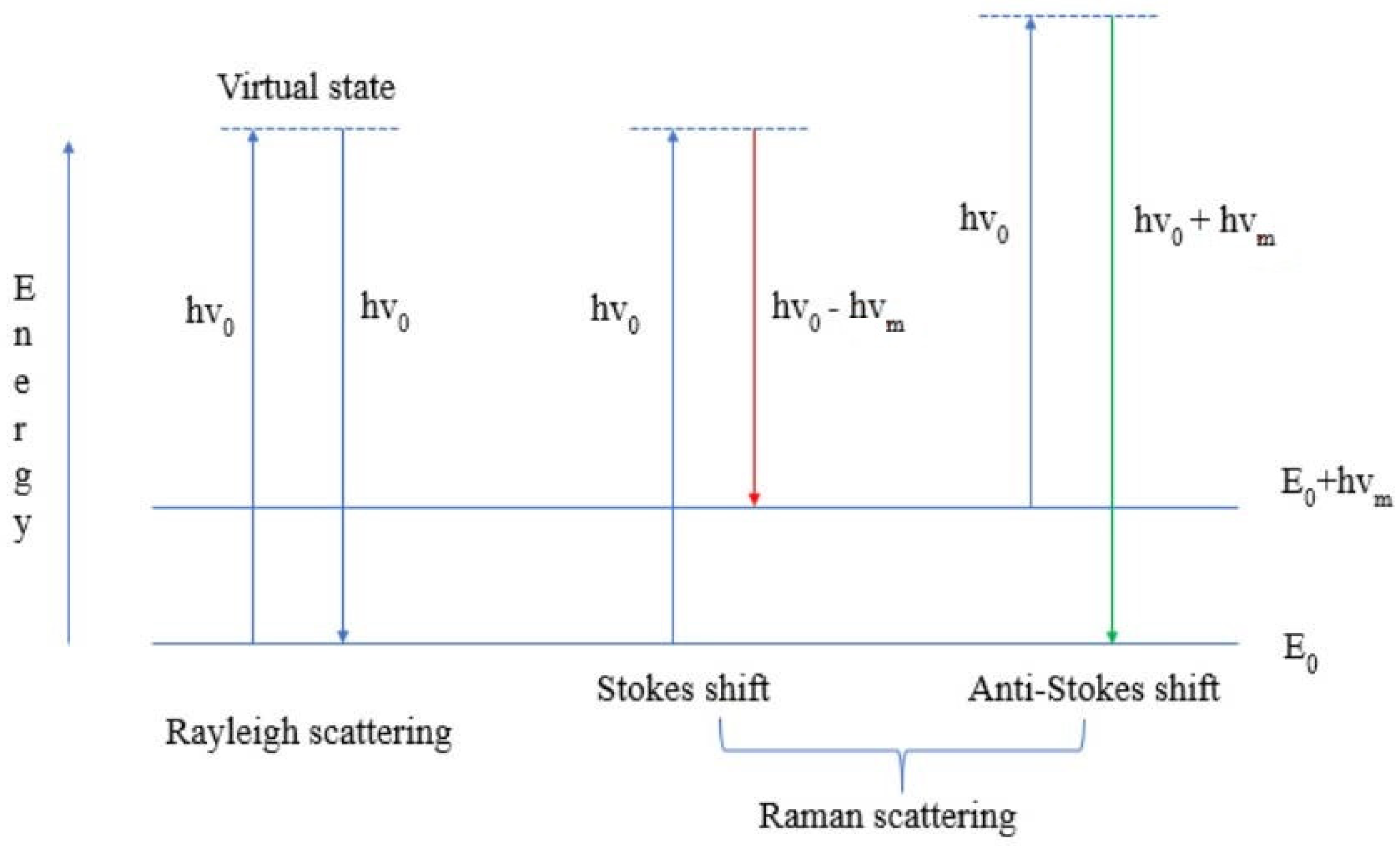
2. Raman Spectroscopy and Its Modifications: Advantages and Use
| Modification of Method | Object | Biomolecules | Reference/Link Number |
|---|---|---|---|
|
Coherent anti-Stokes Raman scattering (CARS) and microscopy |
microalgae |
lipids, carotenoids |
|
|
Confocal Raman microscopy |
microalgae, algae |
lipids |
|
|
Raman micro spectroscopy |
algae, animals |
lipids, carotenoids |
|
|
Resonance Raman spectroscopy (RRS) |
bacteria, microalgae |
carotenoids |
|
|
Single-cell Raman spectroscopy (SCRS) |
microalgae |
lipids |
|
|
Surface-enhanced Raman spectroscopy (SERS) |
animals, bacteria, microalgae |
lipids, carotenoids, proteins |
2.1. Surface-Enhanced Raman Spectroscopy (SERS)
2.2. Coherent Anti-Stokes Raman Scattering (CARS)
2.3. Resonance Raman Spectroscopy (RRS)
2.4. Spatially Offset Raman Spectroscopy (SORS)
3. Raman Spectroscopy for Photosynthetic Studies
4. Raman Spectroscopy for Analytical Studies
This entry is adapted from the peer-reviewed paper 10.3390/cells11030386
References
- Kevin Buckley; Alan G. Ryder; Applications of Raman Spectroscopy in Biopharmaceutical Manufacturing: A Short Review. Applied Spectroscopy 2017, 71, 1085-1116, 10.1177/0003702817703270.
- Ramón A. Alvarez-Puebla; Luis M. Liz-Marzán; SERS-Based Diagnosis and Biodetection. Small 2010, 6, 604-610, 10.1002/smll.200901820.
- Stephen D. Hudson; George Chumanov; Bioanalytical applications of SERS (surface-enhanced Raman spectroscopy). Analytical and Bioanalytical Chemistry 2009, 394, 679-686, 10.1007/s00216-009-2756-2.
- Dana Cialla; Anne März; René Böhme; Frank Theil; Karina Weber; Michael Schmitt; Jürgen Popp; Surface-enhanced Raman spectroscopy (SERS): progress and trends. Analytical and Bioanalytical Chemistry 2011, 403, 27-54, 10.1007/s00216-011-5631-x.
- Daniel Jaeger; Christian Pilger; Henning Hachmeister; Elina Oberländer; Robin Wördenweber; Julian Wichmann; Jan H. Mussgnug; Thomas Huser; Olaf Kruse; Label-free in vivo analysis of intracellular lipid droplets in the oleaginous microalga Monoraphidium neglectum by coherent Raman scattering microscopy. Scientific Reports 2016, 6, 35340, 10.1038/srep35340.
- Lillie Cavonius; Helen Fink; Juris Kiskis; Eva Albers; Ingrid Undeland; Annika Enejder; Imaging of Lipids in Microalgae with Coherent Anti-Stokes Raman Scattering Microscopy. Plant Physiology 2015, 167, 603-616, 10.1104/pp.114.252197.
- X. N. He; J. Allen; P. N. Black; Tommaso Baldacchini; X. Huang; H. Huang; L. Jiang; Y. F. Lu; Coherent anti-Stokes Raman scattering and spontaneous Raman spectroscopy and microscopy of microalgae with nitrogen depletion. Biomedical Optics Express 2012, 3, 2896-2906, 10.1364/BOE.3.002896.
- Fisseha Bekele Legesse; Jan Rüger; Tobias Meyer; Christoph Krafft; Michael Schmitt; Jürgen Popp; Investigation of Microalgal Carotenoid Content Using Coherent Anti-Stokes Raman Scattering (CARS) Microscopy and Spontaneous Raman Spectroscopy. ChemPhysChem 2018, 19, 1048-1055, 10.1002/cphc.201701298.
- Sudhir Kumar Sharma; David R. Nelson; Rasha Abdrabu; Basel Khraiwesh; Kenan Jijakli; Marc Arnoux; Matthew J. O’Connor; Tayebeh Bahmani; Hong Cai; Sachin Khapli; et al. An integrative Raman microscopy-based workflow for rapid in situ analysis of microalgal lipid bodies. Biotechnology for Biofuels 2015, 8, 1-14, 10.1186/s13068-015-0349-1.
- Shixuan He; Wanyi Xie; Ping Zhang; ShaoXi Fang; Zhe Li; Peng Tang; Xia Gao; Jinsong Guo; Chaker Tlili; Deqiang Wang; et al. Preliminary identification of unicellular algal genus by using combined confocal resonance Raman spectroscopy with PCA and DPLS analysis. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 2018, 190, 417-422, 10.1016/j.saa.2017.09.036.
- Nadezda A. Brazhe; Andrey B. Evlyukhin; Eugene Goodilin; Anna A. Semenova; Sergey Novikov; Sergey I. Bozhevolnyi; Boris N. Chichkov; Asya S. Sarycheva; Adil A. Baizhumanov; Evelina I. Nikelshparg; et al. Probing cytochrome c in living mitochondria with surface-enhanced Raman spectroscopy. Scientific Reports 2015, 5, 13793, 10.1038/srep13793.
- Kateřina Osterrothová; Adam Culka; Kateřina Němečková; David Kaftan; Linda Nedbalová; Lenka Prochazkova; Jan Jehlicka; Analyzing carotenoids of snow algae by Raman microspectroscopy and high-performance liquid chromatography. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 2019, 212, 262-271, 10.1016/j.saa.2019.01.013.
- Jan Jehlicka; Howell G. M. Edwards; Kateřina Osterrothová; Julie Novotná; Linda Nedbalová; Jiří Kopecký; Ivan Němec; Aharon Oren; Potential and limits of Raman spectroscopy for carotenoid detection in microorganisms: implications for astrobiology. Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences 2014, 372, 20140199, 10.1098/rsta.2014.0199.
- Elizabeth Kish; Ke Wang; Manuel J. Llansola-Portoles; Cristian Ilioaia; Andrew A. Pascal; Bruno Robert; Chunhong Yang; Probing the pigment binding sites in LHCII with resonance Raman spectroscopy: The effect of mutations at S123. Biochimica et Biophysica Acta (BBA) - Gene Regulatory Mechanisms 2016, 1857, 1490-1496, 10.1016/j.bbabio.2016.06.001.
- Tingting Wang; Yuetong Ji; Yun Wang; Jing Jia; Jing Li; Shi Huang; Danxiang Han; Qiang Hu; Wei E Huang; Jian Xu; et al. Quantitative dynamics of triacylglycerol accumulation in microalgae populations at single-cell resolution revealed by Raman microspectroscopy. Biotechnology for Biofuels 2014, 7, 58-58, 10.1186/1754-6834-7-58.
- Huawen Wu; Joanne V. Volponi; Ann E. Oliver; Atul N. Parikh; Blake A. Simmons; Seema Singh; In vivo lipidomics using single-cell Raman spectroscopy. Proceedings of the National Academy of Sciences 2011, 108, 3809-3814, 10.1073/pnas.1009043108.
- Evelina I. Nikelshparg; Vera G. Grivennikova; Adil A. Baizhumanov; Anna A. Semenova; Victoria Sosnovtseva; Eugene A. Goodilin; Georgy V. Maksimov; Nadezhda A. Brazhe; Probing lipids in biological membranes using SERS. Mendeleev Communications 2019, 29, 635-637, 10.1016/j.mencom.2019.11.009.
- Ota Samek; Alexandr Jonáš; Zdeněk Pilát; Pavel Zemanek; Ladislav Nedbal; Jan Tříska; Petr Kotas; Martin Trtílek; Raman Microspectroscopy of Individual Algal Cells: Sensing Unsaturation of Storage Lipids in vivo. Sensors 2010, 10, 8635-8651, 10.3390/s100908635.
- Shijie Fu; Xiwen Wang; Ting Wang; Zhiping Li; Deming Han; Chunsheng Yu; Cui Yang; Han Qu; Hang Chi; Yutian Wang; et al. A sensitive and rapid bacterial antibiotic susceptibility test method by surface enhanced Raman spectroscopy. Brazilian Journal of Microbiology 2020, 51, 875-881, 10.1007/s42770-020-00282-5.
- Yu-Luen Deng; Yi-Je Juang; Black silicon SERS substrate: Effect of surface morphology on SERS detection and application of single algal cell analysis. Biosensors and Bioelectronics 2014, 53, 37-42, 10.1016/j.bios.2013.09.032.
- Wang, Y. Construction of Artificial Intelligence-Assisted Prostate Tumor Early Diagnosis System Based on Surface Enhanced Raman Spectroscopy. 2020. Available online: http://www.chictr.org.cn/showproj.aspx?proj=60141 (accessed on 12 December 2021).
- U.S. National Library of Medicine Non-Invasive Assessment of Mechano-Chemical Properties of Urine Proteins by Hybrid Brillouin-Raman Spectroscopy. Available online: https://clinicaltrials.gov/ct2/show/NCT04311684 (accessed on 12 December 2021)
- U.S. National Library of Medicine. Raman Analysis of Saliva as Biomarker of COPD (BIO-RAnCh). Available online: https: //clinicaltrials.gov/ct2/show/NCT04628962 (accessed on 12 December 2021)
- Cristina L. Zavaleta; Ellis Garai; Jonathan T. C. Liu; Steven Sensarn; Michael J. Mandella; Dominique Van de Sompel; Shai Friedland; Jacques Van Dam; Christopher H. Contag; Sanjiv S. Gambhir; et al. A Raman-based endoscopic strategy for multiplexed molecular imaging. Proceedings of the National Academy of Sciences 2013, 110, E2288-E2297, 10.1073/pnas.1211309110.
- Chunhuan Jiang; Ying Wang; Wei Song; Lehui Lu; Delineating the tumor margin with intraoperative surface-enhanced Raman spectroscopy. Analytical and Bioanalytical Chemistry 2019, 411, 3993-4006, 10.1007/s00216-019-01577-9.
- Conor L. Evans; X. Sunney Xie; Coherent Anti-Stokes Raman Scattering Microscopy: Chemical Imaging for Biology and Medicine. Annual Review of Analytical Chemistry 2008, 1, 883-909, 10.1146/annurev.anchem.1.031207.112754.
- Conor L. Evans; Eric O. Potma; Mehron Puoris'Haag; Daniel Côté; Charles P. Lin; X. Sunney Xie; Chemical imaging of tissue in vivo with video-rate coherent anti-Stokes Raman scattering microscopy. Proceedings of the National Academy of Sciences 2005, 102, 16807-16812, 10.1073/pnas.0508282102.
- Ji-Xin Cheng; X. Sunney Xie; Coherent Anti-Stokes Raman Scattering Microscopy: Instrumentation, Theory, and Applications. The Journal of Physical Chemistry B 2003, 108, 827-840, 10.1021/jp035693v.
- Akshay Sood; Wooju Jeong; James O. Peabody; Ashok K. Hemal; Mani Menon; Robot-Assisted Radical Prostatectomy. Urologic Clinics of North America 2014, 41, 473-484, 10.1016/j.ucl.2014.07.002.
- Masaya Okada; Nicholas Isaac Smith; Almar Flotildes Palonpon; Hiromi Endo; Satoshi Kawata; Mikiko Sodeoka; Katsumasa Fujita; Label-free Raman observation of cytochrome c dynamics during apoptosis. Proceedings of the National Academy of Sciences 2011, 109, 28-32, 10.1073/pnas.1107524108.
- M. Z. Vardaki; C. G. Atkins; H. G. Schulze; D. V. Devine; K. Serrano; M. W. Blades; R. F. B. Turner; Raman spectroscopy of stored red blood cell concentrate within sealed transfusion blood bags. The Analyst 2018, 143, 6006-6013, 10.1039/c8an01509k.
- Guanping Feng; Marien Ochoa; Jason R. Maher; Hani Awad; Andrew J. Berger; Sensitivity of spatially offset Raman spectroscopy (SORS) to subcortical bone tissue. Journal of Biophotonics 2017, 10, 990-996, 10.1002/jbio.201600317.
- Kumud Bandhu Mishra; Petr Vítek; Anamika Mishra; Josef Hájek; Miloš Barták; Chlorophyll a fluorescence and Raman spectroscopy can monitor activation/deactivation of photosynthesis and carotenoids in Antarctic lichens. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 2020, 239, 118458, 10.1016/j.saa.2020.118458.
- Sijia Liu; Akash Kannegulla; Xianming Kong; Ran Sun; Ye Liu; Rui Wang; Qian Yu; Alan X. Wang; Simultaneous colorimetric and surface-enhanced Raman scattering detection of melamine from milk. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 2020, 231, 118130, 10.1016/j.saa.2020.118130.
- Rustin Mirsafavi; Martin Moskovits; Carl Meinhart; Detection and classification of fentanyl and its precursors by surface-enhanced Raman spectroscopy. The Analyst 2020, 145, 3440-3446, 10.1039/c9an02568e.
- Kumud Bandhu Mishra; Petr Vítek; Anamika Mishra; Josef Hájek; Miloš Barták; Chlorophyll a fluorescence and Raman spectroscopy can monitor activation/deactivation of photosynthesis and carotenoids in Antarctic lichens. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 2020, 239, 118458, 10.1016/j.saa.2020.118458.
- Sijia Liu; Akash Kannegulla; Xianming Kong; Ran Sun; Ye Liu; Rui Wang; Qian Yu; Alan X. Wang; Simultaneous colorimetric and surface-enhanced Raman scattering detection of melamine from milk. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 2020, 231, 118130, 10.1016/j.saa.2020.118130.
- Rustin Mirsafavi; Martin Moskovits; Carl Meinhart; Detection and classification of fentanyl and its precursors by surface-enhanced Raman spectroscopy. The Analyst 2020, 145, 3440-3446, 10.1039/c9an02568e.
