The release of extracellular vesicles (EVs) is a common language, used by living organisms from different kingdoms as a means of communication between them. Extracellular vesicles are lipoproteic particles that contain many biomolecules, such as proteins, nucleic acids, and lipids. The primary role of EVs is to convey information to the recipient cells, affecting their function. Plant-derived extracellular vesicles (PDEVs) can be isolated from several plant species, and the study of their biological properties is becoming an essential starting point to study cross-kingdom communication, especially between plants and mammalians.
1. Introduction
The constant communication between all living organisms, such as plants, bacteria, and animals, attracts the scientific interest of the biomedical community. In general, lipids, proteins, and nucleic acids can be transferred through extracellular vesicles (EVs), which are shuttles involved in cell-to-cell communication.
Extracellular vesicles are lipoproteic particles released by organisms belonging to different kingdoms, and research in recent years has indicated they are mainly involved in cross-kingdom interaction.
The content of EVs consists of various biomolecules, such as proteins, nucleic acids, sugars, and lipids. The main role of EVs is to transfer information to the receiving cells, thereby influencing their functions [
1]. Among EVs, different subtypes can be recognized; the International Society for Extracellular Vesicles periodically publishes new guidelines for EV research (MISEV guidelines), and in the latest recommendation, EV subtypes are distinguished based on their physical properties, such as dimension and density (small EVs or medium/large EVs), the biochemical composition (CD63+/CD81+—EVs, Annexin A5-stained EVs, etc.), and on the cell of origin [
1]. The discovery of the presence of both messenger and small noncoding RNAs inside mammalian EVs in 2007 revolutionized the field of cell–cell communication [
2]. Many studies correlated the ability of EVs to modulate the functional properties of target cells with their RNA cargo [
3,
4,
5]. RNAs contained in EVs, once internalized, can trigger molecular and phenotypic changes in the recipient cell. EV-mRNAs can be translated into functional proteins, while EV-ncRNAs can engage complex networks of interactions that regulate gene expression [
6]. For instance, the EVs released by multiple myeloma cells are enriched in some miRNAs that can affect the expression of genes involved in osteogenic differentiation in mesenchymal stem cells. Among them, miR-129-5p downregulates the expression of SP1, a positive regulator of osteogenic differentiation, and alkaline phosphatase in mesenchymal stem cells; thus, inhibiting osteoblastic differentiation [
3]. RNAs delivered by EVs are also involved in pre-metastatic niche formation. Conigliaro et al. demonstrated that EVs released by CD90+ liver cancer cells carry lncRNA H19, which promotes tube formation and the cell–cell interaction of endothelial cells; thus, favoring angiogenesis [
7]. EV-RNAs play a key role, not only in pathological, but also in physiological, processes; understanding their mechanisms of action could lead to new therapeutic strategies. Li and colleagues demonstrated the beneficial effects of EVs isolated from M2 microglia cells in mice with ischemic stroke. They found that M2 microglial EVs were enriched in miR-124, which suppresses astrocytes proliferation through the inhibition of the STAT3 pathway; thus, reducing glial scar formation [
8].
2. The Story of Plant EVs, from Their Discovery to the Purification from Different Matrices
Although new techniques for PDEVs isolation have emerged in the last year, such as immunocapture purification [
28] and the aqueous two-phase system [
49], differential centrifugation remains the most widely used method [
40,
46,
50]. Some groups adopted isolation protocols similar to those used for mammalian EVs. Briefly, the starting material is squeezed, and the obtained juice is subjected to multiple steps of centrifugation: low-speed centrifugation (500–3000×
g for about 10 min), intermediate speed centrifugation (2000–10,000×
g for about 30 min), and ultracentrifugation (100,000–150,000×
g for 1.5–2 h) to obtain a PDEV pellet. However, since ultracentrifugation also sediments other vesicles, proteins, and protein/RNA aggregates; while, some protocols add a subsequent density gradient ultracentrifugation using iodixanol or sucrose/deuterium oxide to separate PDEVs from contaminants [
50,
51,
52]. On the other hand, Regente and other researchers have found that PDEVs can pellet at a lower speed, such as 40,000×
g [
21,
26,
53].
Once isolated, PDEVs should be appropriately characterized using various methods, including nanoparticle tracking, flow-cytometry, transmission electron microscopy, and other forms of electron microscopy. The PDEV cargo is complex and heterogeneous, for this reason, the best methods for studying their content are based on omics analysis. In the last years, several studies were carried out to characterize the proteomic profile of EVs isolated from different plant species [
10,
21,
26,
30,
42,
54]; these findings could help to identify possible PDEVs markers. Among the proteins identified in PDEVs, the most represented are heat shock protein 70 (HSP70), S-adenosyl-homocysteinase, and glyceraldehyde 3 phosphate dehydrogenase, which were found in EVs isolated from olive pollen [
55],
Nicotiana benthamiana [
56],
Arabidopsis thaliana [
26], and sunflower [
25]; these proteins could be candidates as PDEVs markers, even if further studies are needed to confirm their presence in EVs isolated from the majority of plants. Other protein families well represented in PDEVs are that of aquaporins, present in citrus- [
10,
30,
52] and grape-derived EVs [
56]; and annexins, found in citrus- [
10,
30], sunflower- [
21], and Arabidopsis-derived EVs [
26]. Besides proteins, the lipid characterization of PDEVs has attracted the interest of the scientific community, since this component seems to be strictly correlated to their biological functions [
23]. The main lipid species identified in PDEVs are phosphatidic acid (PA), found in EVs from sunflower [
21], grape [
56], ginger [
23],
Uvae-ursi folium,
Craterostigma plantagineum, and
Zingiberis rhizome [
57]; phosphatidylethanolamine (PE), described in EVs-derived from grapefruit [
58], grape [
56],
Craterostigma plantagineum, and
Zingiberis rhizome [
57]; and phosphatidylcholine (PC), present in grapefruit- [
58] and
Craterostigma plantagineum-EVs [
57]. Moreover, PDEV cargo includes several metabolites, such as sulforaphane [
59], shogaol [
60], and flavonoids [
36,
56,
58], which could explain the PDEV-mediated beneficial roles in plant–mammalian interactions. On the other hand, a recent study published by Stanly and colleagues showed that both micro-and nano-vesicles isolated from strawberry carry functional allergens, including Fra a 1, Fra a 3, and Fra a 4 [
51]; thus, demonstrating for the first time that PDEVs can also transport this type of molecule.
PDEVs also contain different species of RNA; this topic and the correlated studies will be discussed in the following paragraphs.
3. Functional Role of PDEV-RNAs in Cross-Kingdom Interactions
Discoveries correlated to plant-derived extracellular vesicles are becoming an essential starting point to study cross-kingdom communication; as a consequence, several studies have been carried out to analyze the interaction between plant vesicles and mammalian targets (Figure 4).
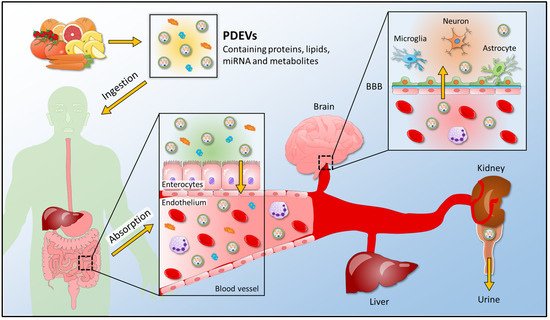
Figure 4. A schematic view for the uptake of plant-derived extracellular vesicles into the human body. PDEVs containing proteins, lipids, miRNA, and metabolites enter the human body after edible plant ingestion. In the gastrointestinal tract, where food is digested, PDEVs are absorbed and enter the bloodstream; thus, reaching the final recipient organs, such as the brain, liver, and kidney. PDEVs release their content in target organs and exert their biological properties. They can cross the BBB and reach the cells of the central nervous system, or they can be found in the urine of plant-eating humans. PDEVs, plant-derived extracellular vesicles; BBB, blood-brain barrier.
3.1. Functional Roles of Plant Extracellular Vesicles in Plant-Mammalian Communication
MiRNAs’ role in the anti-inflammatory effect of PDEVs has attracted many researchers: several studies were carried out to find the correlation between miRNAs packed into plant-derived extracellular vesicles and inflammatory response targets. For example, an interesting finding reports that mammalian genes involved in the regulation of inflammatory cytokines (IL-5 and IL-6) can be targeted by PDEV miRNAs, suggesting that these plant vesicle-derived miRNAs can potentially regulate mammalian mRNAs and biological pathways. miRNAs can, indeed, directly target genes encoding inflammatory factors. For example, miR-5781 in soybean-EVs can directly target
IL-17A. MiR-4995 packed into tomato-EVs can target
IL-5, while miR-1078 in ginger-EVs can target
IL-6 [
27]. Consequentially, it is important to consider the potential roles that these PDEV-derived miRNAs can play in host health and disease, paving the way for new cross-kingdom interaction findings [
86]. Interestingly, a therapeutic effect of plant miRNAs in the prevention of chronic inflammation was shown in a mouse model of human multiple sclerosis. In particular, the impact of plant miRNAs on dendritic cells was explored, a component of the innate immune system in the gut and responsible for instructing T cells.
Fragaria vesca miR-168 can reduce the inflammation mediated by TLR agonists via a TLR3-mediated mechanism: treatment with plant miRNA can reduce inflammation and prevent symptoms of multiple sclerosis in mouse models. In particular, it was demonstrated that miR-168 can reduce the inflammatory response, and this effect was associated with a decreased expression of TRIF transcript, an essential adaptor protein required for innate immune responses mediated by TLR3 [
87]. Moreover, Link et al. found detectable levels of plant miR-168 in human feces, normal gastric, and colon cancer mucosa [
88], suggesting potential interspecies activity.
Another role mediated by PDEVs is their antioxidant effect on human cell models. An interesting study reported the antioxidant effect of strawberry-derived extracellular vesicles. These EVs were isolated from the strawberry juice of
Fragaria x ananassa and they could prevent oxidative stress in human mesenchymal stromal cells in a dose-dependent manner. The analysis of their cargo revealed the presence of small RNAs and miRNAs; in particular, a specific enrichment of miR-166g was found [
89]. The antioxidant role of PDEVs has also been demonstrated, thanks to the analysis of extracellular vesicles derived from other plant species. For example, carrot-derived EVs were investigated for their antioxidative and apoptotic effects in cardiomyoblasts and neuroblastoma cells. Carrot-derived EVs can inhibit the ROS generation induced by H
2O
2 and apoptosis induction. In particular, Kim et al. reported that carrot-derived EVs can inhibit the decrease in
Nrf-2 expression in cardiomyoblast cells, thereby protecting cells from oxidative stress. In contrast, the decrease in
HO-1 expression is reduced when cells are supplemented with carrot-derived EVs. Similar expression patterns are observed for
NQO-1 expression, indicating that carrot-derived EVs effectively inhibit the decrease in the expression of this antioxidative protein [
90].
3.2. Plant Extracellular Vesicles in Plant-Microbe Interaction
Plants and animals can be under constant pathogen attack. Some pathogens and pests deliver small RNAs (sRNAs) into host cells to suppress host immunity. Conversely, hosts also transfer sRNAs into pathogens and pests to inhibit their virulence. Emerging findings have revealed that some sRNAs can, indeed, travel between hosts and interact with microbes and fungi to silence target genes. In particular, RNA interference (RNAi) is one of the primary adaptive defense mechanisms that can regulate plant immune responses against several kinds of pathogens. For example, plants such as cotton can export plant-specific miRNAs into their fungal pathogens to induce cross-kingdom gene silencing and confer disease resistance. Cotton plants produce miR-166 and miR-159, which are exported to the hyphae of pathogenic fungus
Verticillium dahlia. These miRNAs target a Ca
2+—dependent cysteine protease and an isotrichodermin C-15 hydroxylase that are crucial for virulence [
91].
Intriguingly, PDEV secretion is increased by pathogen infection, suggesting that EVs play important roles in plant immunity [
26]. In addition to mammalians, plant-derived EVs can, indeed, mediate the communication with pathogens through RNAi. In particular, emerging studies have shown that sRNA derived from plants can silence microorganism and fungi target genes [
92]. As a consequence, the discovery of EV-mediated trafficking of sRNAs from plant hosts to fungal pathogens has given rise to many exciting questions. For instance, Cai et al. discovered that small interfering RNAs are delivered by Arabidopsis into
B. cinerea cells, promoting the silencing of fungal genes [
43]. Further studies have revealed that cross-kingdom RNA trafficking, from the host into the pathogen, to induce the silencing of pathogenic genes, depends on EVs [
93,
94]. Arabidopsis sRNAs transported into
B. cinerea cells are also packed into plant-derived EVs, indicating that EV-mediated transport is one of the most relevant pathways for the cross-kingdom trafficking of sRNA. Cai et al. also demonstrated that sRNA-containing vesicles accumulate at the infection sites and are taken up by the fungal cells. Moreover, transferred host sRNAs induce silencing of fungal genes critical for pathogenicity. Consequently, it was reported that plant extracellular vesicles play an essential role in cross-kingdom sRNA trafficking between Arabidopsis and the fungal pathogen
B. cinerea, because Arabidopsis secretes EVs to deliver host sRNAs into fungal cells to silence virulence-related genes [
43].
This entry is adapted from the peer-reviewed paper 10.3390/membranes12040352