2.1. Acquisition
Liberibacter interaction with psyllids follows a systematic pattern of acquisition, systemic infection, propagation and circulative transmission (
Figure 2).
Liberibacter acquisition by the psyllids is highly variable, and may be influenced by the psyllid’s developmental stage and factors affecting the life cycle of the host insect. In addition, environmental factors may also affect acquisition. The acquisition rates of
Liberibacter may vary from 1–90% [
40].
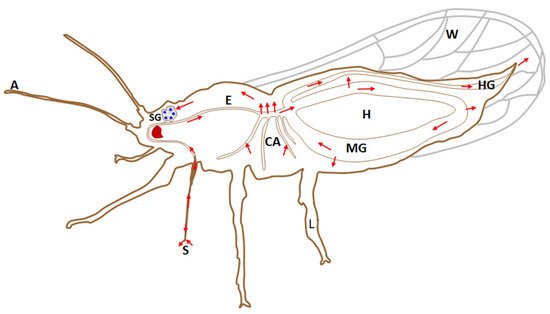
Figure 2. A graphical illustration of the internal anatomy and organs of an adult psyllid, showing the circulative pathway that bacteria of the genus Liberibacter pass through during the transmission process. Red arrows show the pathway, which starts with acquisition from an infected plant through the stylet (S), moves along the esophagus (E), reaches the midgut (MG), where the bacterium is absorbed into the hemolymph (H), circulates and reaches the salivary glands (SG), from which it is secreted into the newly infected plant through the salivary canal in the stylet. A, antennae; CA, caeca; W, wings; HG, hindgut; L, leg.
The acquisition, as well as the transmission of
Liberibacter, is dependent on an Acquisition Access Period (AAP), i.e., the time required to acquire the bacterial pathogen by the psyllid feeding on a
Liberibacter-infected host plant [
41]. The psyllids may acquire
Liberibacter as adults or as nymphs (
Figure 3). Sengoda et al. [
25] studied the effect of different AAP on CLso acquisition by adult psyllids exposed to infected potato plants. The study showed that the potato psyllid (
B. cockerelli) adults could successfully acquire CLso from the infected plants after an AAP as short as 4 h. Also, the CLso titer in psyllids increased over time following AAPs of 8, 24 and 72 h, and reached a plateau after an average of 15 days. The CLso acquisition rate at plateau was 35% (24 h AAP) and 80% (72 h AAP). Tang et al. [
42] observed distinct acquisition and transmission rates for CLso haplotypes (CLso A and CLso B). The titer of CLso B increased rapidly, to reach a plateau after 6 days, while CLso A titer augmented slowly to plateau after 16 days. Additionally, CLso B showed a shorter latent period (17–21 days, after 7 days AAP) than CLso A (21–25 days, after 7 days AAP). Inoue et al. [
43] reported a CLas acquisition rate of 88% in
D. citri adults after 24 h AAP. The proportion of the CLas-positive psyllid population declined to 50% at 20 d post-acquisition.
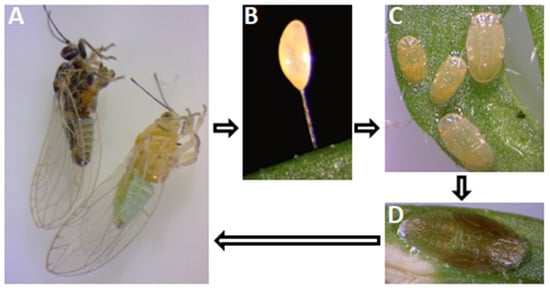
Figure 3. Life cycle of the carrot psyllid. Adult females (A) lay eggs on the plant leaves and stems (B). The eggs are supported in the plant tissue by a pedicel. The eggs hatch and development passes through several nymphal stages (C,D) before adult emergence (A).
Nymphs were reported to have better acquisition efficiency, compared to adult psyllids. Ammar et al. [
44] studied the effects of AAP by the nymphs and adults of
D. citri on CLas acquisition, multiplication and transmission. They reported that following one or seven days of AAP as nymphs, 49–59% of CLas-exposed psyllids became CLas-infected, whereas infected adults amounted to 8–29%. Furthermore, CLas titer in the CLas-exposed psyllids was significantly higher, and increased at a faster rate (reaching a peak at 14–28 days after first being exposed to diseased plants). The CLas acquisition by adult psyllids (reached a peak at 21–35 days after first being exposed to diseased plants) was comparatively slower, with CLas titer decreasing, or fluctuating, after reaching a peak in both nymphs and adults. The CLas titer was higher with an increase in AAP, especially regarding acquisition as adults. Similar observations of
D. citri nymphs being more efficient in CLas acquisition than adult psyllids were made by Wu et al. [
40]. In this study, the nymphs were able to acquire CLas within 30 min of feeding, while adult insects needed a minimum of 5 h of feeding to acquire the pathogen. The bacterial titers in the alimentary canal, hemolymph and salivary glands of the infected psyllids showed variable trends. After acquisition, CLas titers in the alimentary canal of infected nymphs and adults declined over the course of experimental period, with the decrease more pronounced in adults than in nymphs. CLas titers in the hemolymph of nymphs was initially high, then declined, before rising again, while for the adult insects, the bacterial titer decreased rapidly to zero. In the salivary glands of the nymphs, the proportion of infected glands, as well as CLas titer in the glands, gradually increased throughout the experiment, whereas for the adults, both the proportions and titers remained low. Brlansky and Rogers [
33] reported CLas acquisition at 60–100% in
D. citri nymphs, while only 40% acquisition was observed in adults after 5 weeks of feeding on the infected plants. In another study by Pelz-Stelinski et al. [
8], efficiency of nymphs in CLas acquisition was 60–100%, compared to adults at 40%, re-emphasising the fact that the nymphs were more likely to acquire CLas from infected plants [
8]. However, a decline in pathogen titer over time was observed in both nymphs and adults. Inoue et al. [
37] reported a significant decline in the percentage of CLas-positive adults (from 50% after 20 days AAP to 13% after 42 days AAP), which was different from earlier reports where CLas-positive adults ranged from 55–70% after 42 days AAP [
45]. The decline may be attributed to the host aging, and/or negative effects of the bacterial infection in the form of deteriorated resources in the insect host. Inoue et al. [
43] alluded to the reduction in CLas-positive psyllids as being due to the death of positive psyllids and/or a temporal infection shift from being positive to negative because of the excretion of the CLas population from the alimentary canal. These studies indicate that there are significant differences in the biology of
Liberibacter acquisition and multiplication between different species of psyllids. However, it is to be noted that no differences in CLas acquisition, or titers, was found between male and female
D. citri, omitting the possibility of sex-based preference for the vector [
40].
The host plant species and the bacterial titer in the exposed plants may also affect acquisition and titers within the vector. Sengoda et al. [
41] showed that potato psyllids acquire the bacteria faster and in higher titer, when exposed to CLso-infected tomato plants than potato plants. Following an AAP of 72 h, the CLso-acquisition rate in psyllids fed on potato and tomato was 80 and 100%, respectively, while the CLso titer was 200 to 400-fold higher when psyllids acquired CLso from the tomato plants than from the potato plants. However, the acquisition of CLas by
D. citri was found to be independent of host plant genotype [
40]. Nevertheless, a feeding preference was apparent in the insects, which was likely to increase the possibility of acquiring the pathogen from the preferred plants. The CLas titer in the psyllids increased with increase in duration of feeding. The leaf age and disease severity (bacterial titers) in the plants appeared to affect CLas acquisition more than the genotype. Feeding by adult
D. citri declines markedly with increase in leaf age, thus diminishing the possibility of CLas acquisition.
Abiotic factors, such as temperature, humidity, and rainfall, influence
Liberibacter acquisition by influencing plant growth parameters. CLas titers in CLas-infected plants declined with an increase in ambient and leaf temperatures. Consequently, the proportion of infected population, as well as CLas titer in the infected adults, shows a gradual decrease with increase in ambient temperatures from 24 to 38 °C [
40]. This indicates a direct relationship between CLas titer in plants and the proportion of psyllids acquiring CLas and their acquisition efficiency. Psyllids kept at 25 °C not only showed a higher proportion of psyllids with CLas acquisition, but also the highest titers of bacteria in the infected insects. In field conditions on a Florida farm, maximum acquisition was recorded between September to October, when average temperatures were 22–27 °C, with maxima below 32 °C [
40,
46]. Temperatures below 17 °C or higher than 32 °C were deemed to be detrimental to the survival of CLso [
47,
48]. The differences in acquisition due to temperature may reflect the following: (i) better growth of bacteria in the host plants at 25 °C, leading to high bacterial titer acquired by the insects; (ii) 25 °C being the optimum temperature to stimulate psyllid feeding behaviour, facilitating CLas acquisition; (iii) optimal growth of bacteria within psyllid hosts post-acquisition is at 25 °C. Any of these factors alone, or in combination, may play an indirect role in influencing
Liberibacter acquisition by their insect hosts. However, the direct effect of temperatures on
Liberibacter acquisition by psyllids is debatable.
2.2. Systemic Infection
Liberibacter is persistently transmitted and capable of colonising a majority of psyllid tissues, including reproductive organs (
Figure 2) [
4]. A persistent pathogen, once acquired by the nymph or adult insect, remains in the vector for a long time. After being acquired by the psyllids, these pathogens first colonise the psyllid gut (
Figure 4). After replicating in the gut,
Liberibacter proceed to the hemolymph and infect other psyllid tissues, including the salivary glands (
Figure 4), prior to their injection into the host plants during subsequent feeding [
42]. This pattern of infection of organs and tissues of the insect in a propagative manner is termed systemic infection.
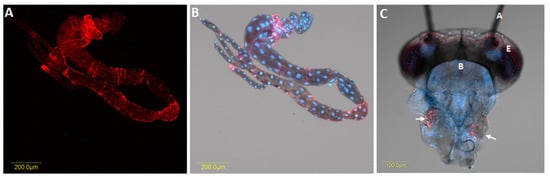
Figure 4. Localisation of Liberibacter solanacearum (CLso) in the midgut of an adult psyllid (A, B) and in salivary glands (C), using fluorescence in situ hybridisation (FISH). A (dark field), B (bright field) and C (bright field of an adult head) show the localisation of CLso (red) as a stripe-like pattern in the midgut and a scattered pattern in the salivary glands (white arrows). Blue in all images is DAPI staining of the nuclei. A, antennae; E, eyes; B, brain.
The investigation of
Liberibacter in specific organs of psyllid is critical for understanding the epidemiology of this pathogen. After acquisition by psyllids,
Liberibacter can be detected in the alimentary canal, malpighian tubules, hemolymph, salivary glands, fat tissue and ovaries; indicating systemic colonisation [
46] (
Figure 4). Although the probability of
Liberibacter infection differed significantly among the different organs of both adults and nymphs, the possibility of their occurrence in each specific organ did not differ between male and female insects, regardless of the insect’s life stage [
37]. Interestingly, irrespective of life stage,
Liberibacter was observed more often in the alimentary canal, compared with other organs. Conversely, the proportion of infected salivary glands was found to be significantly lower. Cooper et al. [
37] studied the prevalence of CLso in the hemolymph, bacteriomes, alimentary canals, and salivary glands of nymphs and adults of
B. cockerelli. In their study, adult psyllids contained 66% (alimentary canals), 39% (salivary glands), and 40% (bacteriomes) of
Liberibacter, while the fifth instar nymphs harboured relatively less
Liberibacter at 52% (alimentary canals), 10% (salivary glands), and 6% (bacteriomes). Ammar et al. [
27] investigated CLas titer in the dissected organs of individual
D. citri adults. They reported
Liberibacter at 72–80%, 47–70%, and 79–97.5% in the alimentary canal, salivary glands, and the rest of the insect body, respectively. However, the proportion of bacteria in the salivary glands was comparatively lower than in other parts of the psyllid body, suggesting existence of a barrier to bacterial movement into salivary glands.
Post-acquisition, psyllids have been shown to retain
Liberibacter in their bodies for most of their lifespans [
49].
D. citri maintained CLas-carrying ability for 12 weeks (84 days), indicating pathogen persistence. Contradictorily, Pelz-Stelinski et al. [
8] proposed that
Liberibacter was not very stable in adult psyllids, on account of their observation of a decrease in number of CLas-positive psyllids over time after bacterial acquisition. Similarly, systemic infection may reflect ambiguity in different pathogens and insect hosts. For instance, CLeu was detected in the midgut and salivary glands of
Cacopsylla pyri, but not in the reproductive organs [
27].
Multiplication of
Liberibacter inside the psyllid host is poorly understood, with conflicting evidence on pathogen replication [
5,
7]. The life stage at which the psyllid becomes infected appears to affect subsequent multiplication. Gradual decrease of CLas-infected psyllids with increase in post-acquisition time, and low transmission efficiency of CLas-exposed psyllids into healthy plants, has been taken as evidence for the non-propagative nature of
Liberibacter [
5,
7,
8,
44]. However, systemic infection of
Liberibacter points out their propagation in the vector [
4,
46]. Adults that acquired CLas during their nymphal stage, showed a very high proportion of infected body parts (80–97.5%) [
46]. Even though the proportion of CLas-infected salivary glands in the insect population was generally lower than the proportion of infected alimentary canals, or other body parts, the relative titer of CLas in the salivary glands and alimentary canals was significantly higher than the rest of the body. The high relative titer of CLas in the alimentary canal and salivary glands was attributed to bacterial replication, accumulation, or both. Inoue et al. [
37] estimated the CLas titer in nymphs and adults of
D. citri after 10-20 days post-acquisition. They reported a significant increase in CLas titer in the nymphs (25-130-fold), but not in the adults, suggesting that
Liberibacter replication in psyllids occurs only when they are acquired by the nymph. Corroborating the previous result, Wu et al. [
24] reported no CLas multiplication in the alimentary canal, hemolymph, and salivary glands of adults exposed to acquisition. However, CLas multiplication was detected in the hemolymph and salivary glands of adults when the bacteria were acquired by nymphs. Nevertheless, the apparent delay, or poor multiplication ability in adult psyllids, cannot be ruled out as the reason for failure to detect CLas titer in these insects. Ammar et al. [
33] advocated that
Liberibacter replication in psyllids is independent of the life stage in which they were acquired. The reasons why
Liberibacter appear to multiply faster, or more efficiently, in psyllid nymphs might be influenced by different feeding behaviours of the nymphs and adults, influence of different symbiotic organisms residing in the nymphs and adults, and nature of the transmission barriers in the gut or salivary glands. The transmission barriers may be more mature or complete in adults, compared to the nymphs.
2.3. Transmission
In most pathogen–vector interactions, successful transmission entails the invasion of one or more organs of the vector, surviving the vector immune response, intra- or extracellular replication, and the development of infectivity prior to transmission [
50]. Successful transmission of
Liberibacter to host plants requires a specific threshold of pathogen titer in the vector [
51,
52]. Consequently, multiplication of
Liberibacter in the psyllids appears to be an essential strategy to achieve threshold titer for efficient transmission. The evidence for requirement of replication within the host for successful transmission was realised when the Mississippi strain of
Anaplasma marginale was unable to establish infection at the level of the midgut epithelium of their vector ixodid ticks, which subsequently prevented their development within salivary glands and transmission [
50].
The transmission efficiency of
Liberibacter by their psyllid hosts appears to be dependent on the life stage of the vector during ingestion [
43,
53]. Pelz-Stelinski et al. [
8] reported that the transmission of CLas by
D. citri adults seems to be most efficient when the bacterium is ingested during the nymphal stages. In their study, adult psyllids exposed to CLas-infected plants (40% tested positive for CLas infection) failed to transmit bacteria to host plants. However, psyllids reared from eggs through to the adult stage (60% tested positive for CLas infection) could successfully transmit (73%) the bacteria to the host plants. The above result implied that when CLas is acquired by an adult host the host failed to transmit the bacteria, and suggested that this was due to the inability of the pathogen to multiply in the adult system. As this claim was later refuted with evidence proving replication of CLas in both nymphs and adults [
44], another possibility emerged that when infection occurs during the adult stage, the time period to reach the adequate bacterial titer for infectivity, or transmission, is not sufficient.
The latent period, the period of time between acquisition and transmission of the pathogen by the insect, represents the amount of time required for the pathogen to translocate from alimentary canal to salivary glands [
41]. The latent period directly correlates to transmission efficiency. The latent period for CLso was reported to be 2-3 weeks in
B. cockerelli [
37].
B. cockerelli adults were found to have better transmission efficiency for CLso, compared to the nymphs [
37,
41,
53,
54]. Although
Liberibacter was observed infecting the salivary glands of both adults (39%) and fifth instar nymphs (10%), the pathogen titer in the psyllid nymphs was significantly lower, indicating that nymphs were less likely to transmit the bacteria than the adults [
37]. It was suggested that bodily attributes of 1st, 2nd and 3rd instar of psyllids were not sufficient to support higher CLso populations, compared to the 5th instar and adults, resulting in a smaller proportion of infective population and reduced transmission efficiency. The discrepancy in
Liberibacter transmission efficiencies among various studies could be an artefact of different experimental conditions used in the transmission assays: number of insects per test plant, duration of acquisition and inoculation access periods, and variety, or physiology, of the test plants, variation in the origin and strain of the pathogen, genotypic variation in the vector, and/or sensitivity of the pathogen detection methods [
55].
In addition to the horizontal transmission route of pathogen–vector–plant, vertical, or transovarial, transmission of pathogens from parent to offspring was also demonstrated. The vertical transmission of
Liberibacter in psyllids occurs at a low rate of 2–6% [
8]. It is indicated as an important survival mechanism for the bacterial pathogens in case of unavailability of suitable plant hosts. The transmission efficiency is subject to the influence of factors, such as environmental conditions, plant defences, and genetic differences among the insect vector in ability to acquire and transmit the pathogen [
7]. However, the existence of proposed barriers within the insect body may play the most significant role in reducing transmission rates of the pathogen, by preventing the movement of
Liberibacter to and from the salivary glands [
7]. Pelz-Stelinski et al. [
56] suggested the presence of a salivary gland escape/exit barrier for CLas in
D. citri, in addition to the salivary gland infection barrier. A barrier was proposed both in the salivary gland and the alimentary canal of the psyllid vector; however, the salivary gland barrier was deemed to be more important in regulating
Liberibacter transmission, compared to the alimentary canal barrier.