Somatic polyploidy was found in the tissues of all multicellular organisms (including algae, mosses, lichens, vascular plants, invertebrates, and vertebrates), which points to its adaptive value. In human and warm-blooded animals, polyploidy can be a part of normal postnatal morphogenetic programs and can be a manifestation of response to pathological stimuli and diseases.
- polyploidy
- evolutionary conserved features
- developmental programming
- cardio-vascular diseases
- transcriptome
1. Ploidy-Associated Transcriptome Features Are Related to Stress Response, Metabolism, Morphogenesis, and Longevity
1.1. Ploidy-Associated Transcriptomic Features Are Evolutionary Conserved
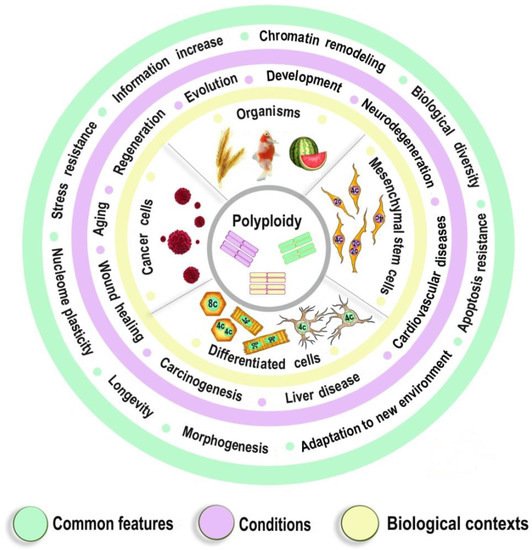
1.2. The Epigenetics of Ploidy-Associated Transcriptomic Features
2. Polyploidy Meets the Hallmarks of Developmental Programming of Adult Diseases in Slowly Renewing or Terminally Differentiated Organs
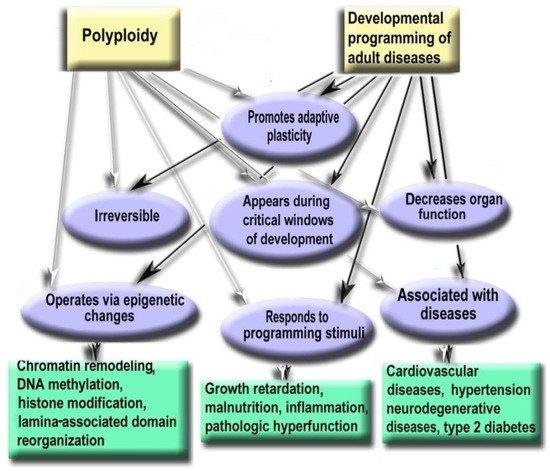
Figure 2. Polyploidy and developmental programming of adult diseases show similar properties.
- Polyploidy helps to cope with the adverse environments via the augmentation of stress resistance and adaptation through epigenetic mechanisms [4][9][54]. Furthermore, it is one of the most variable characteristics of somatic cells. The degree of polyploidization in homologous organs shows large across-species diversity. The percentage of cardiomyocytes with polyploid nuclei varies several folds in mammals of similar weight. For example, about 50% of human cardiomyocytes contain nuclei with 4, 8, 16, or even 32 genomes, whereas cardiomyocytes of the grey wolf or reindeer show only about 1% of cells with polyploid nuclei [55][56]. Accordingly, cardiomyocyte ploidy also varies between individuals of the same species. The mean ploidy in the normal human heart varies from about 4× to 10× [19][57][58]. Thus, polyploidy is characterized by the degree of biologic plasticity similar to the renowned factors of ontogenetic programming.
- Polyploid cells (e.g., cardiomyocytes, megakaryocytes, hepatocytes, pancreacytes, vascular epithelial cells, retina epithelium) appeared in the perinatal and early postnatal ontogenesis [59]. These periods are characterized by high biological plasticity and coincide in time with the critical periods of development [40][41].
- Cells of slowly renewing organs, including neurons of neocortex and cerebellum, cardiomyocytes, and hepatocytes, which accumulate additional genomes in infancy, childhood, and pre-pubertant period, retain the increased genome amount throughout their lives, regardless of environmental conditions [11][19][57][59][60].
- Polyploidization is associated with a decrease in organ functional potential [55][56][61][62]. This decrease probably originates from the involvement of polyploidy in the trade-off between proliferation and function that is also a sign of the developmental programming of adult diseases factor [40][55][63].
- The level of ploidy, particularly in cardiomyocytes, responds to the well-established stimuli of developmental programming (including adverse growth conditions, increased functional load, inflammation, and malnutrition) similarly in the various species and various cells [41][50][55][59]. For example, in mammal hepatocytes, cardiomyocytes, retinocytes, and drosophila somatic cells, polyploidy is associated with the increased response to stress, activated pathways of morphogenesis and glycolytic metabolism, and the weakened aerobic metabolism and apoptosis [9][17][54][64].
- Polyploidy is associated with epigenetic changes at various levels of genome organization leading to chromatin remodeling and genome instability [28][29][33]. The association between polyploidy and chromatin decompactization under stress was well documented for cardiomyocytes and hepatocytes [34][35]. Polyploidy can alter global patterns of DNA methylation, microRNA expression, and histone modification in mammalian, insect, and plant cells [4][9][16][32][33][36][38]. Polyploid cells show higher expression of bivalent genes, which harbor both activating (H3K4me3) and repressive (H3K27me3) chromatin domains, allowing rapid switching between cellular programs [9]. Overall, ploidy-associated transcriptomic changes occur through the same epigenetic mechanisms as in the developmental programming of health and disease, including chromatin remodeling, DNA methylation, histone modification, and others.
- Excessive polyploidization can be associated with the diseases that usually originated from the developmental programming, including cardiovascular disease, hypertension, neurodegenerative disease, type 2 diabetes, metabolic syndrome, and others [18][19][40][41][42][43][59].
Experimental Studies Confirm the Role of Polyploidy in the Developmental Programming of Health and Disease
Recent experimental and clinical studies confirm that polyploidy can be involved in the developmental programming of adult diseases. The most convincing evidence was obtained for cardiovascular diseases that are the most susceptible to developmental programming. Thus, studies in sheep indicated that pre-term birth irreversibly increases the percentage of polyploid mononuclear cardiomyocyte and induces DNA damage, fibrosis, and lymphocytic infiltration [60]. In humans, pathologic hemodynamic load during postnatal growth permanently increases cardiomyocyte ploidy and decreases cardiac performance [33][57][65][66][67][68][69]. The inflammatory stress caused by gastroenteritis in the rat resulted in cardiomyocyte hyperpolyploidization, long-term atrophy, and cell remodeling [59][70]. The experimental model of gastroenteritis was used as gastroenteritis triggers developmental programming factors, including inflammation, growth retardation, and malabsorption, and as gastroenteritis is a major cause of diseases in toddlers, infants, and children [71][72][73]. Both types of neonatal gastroenteritis cause irreversible excessive polyploidization, long-term atrophy, and remodeling of cardiomyocytes [59][74][70]. Altogether, these data indicate that polyploidy can be involved in developmental programming as it is irreversible, responds to programming stimuli during the critical period of development, changes cell phenotype, and weakens cell function, thus meeting all basic criteria of developmental programming.
3. Genome Duplication in Regeneration and Aging
This entry is adapted from the peer-reviewed paper 10.3390/ijms23073542
References
- Anatskaya, O.V.; Vinogradov, A.E. Genome Multiplication as Adaptation to Tissue Survival: Evidence from Gene Expression in Mammalian Heart and Liver. Genomics 2007, 89, 70–80.
- Quinton, R.J.; DiDomizio, A.; Vittoria, M.A.; Kotýnková, K.; Ticas, C.J.; Patel, S.; Koga, Y.; Vakhshoorzadeh, J.; Hermance, N.; Kuroda, T.S.; et al. Whole-Genome Doubling Confers Unique Genetic Vulnerabilities on Tumour Cells. Nature 2021, 590, 492–497.
- Nandakumar, S.; Rozich, E.; Buttitta, L. Cell Cycle Re-Entry in the Nervous System: From Polyploidy to Neurodegeneration. Front. Cell Dev. Biol. 2021, 9, 698661.
- Erenpreisa, J.; Salmina, K.; Anatskaya, O.; Cragg, M.S. Paradoxes of Cancer: Survival at the Brink. In Seminars in Cancer Biology; Academic Press: Cambridge, MA, USA, 2020.
- Lin, H.; Huang, Y.-S.; Fustin, J.-M.; Doi, M.; Chen, H.; Lai, H.-H.; Lin, S.-H.; Lee, Y.-L.; King, P.-C.; Hou, H.-S.; et al. Hyperpolyploidization of Hepatocyte Initiates Preneoplastic Lesion Formation in the Liver. Nat. Commun. 2021, 12, 645.
- Zheng, L.; Dai, H.; Zhou, M.; Li, X.; Liu, C.; Guo, Z.; Wu, X.; Wu, J.; Wang, C.; Zhong, J.; et al. Polyploid Cells Rewire DNA Damage Response Networks to Overcome Replication Stress-Induced Barriers for Tumour Progression. Nat. Commun. 2012, 3, 815.
- Potapova, T.A.; Seidel, C.W.; Box, A.C.; Rancati, G.; Li, R. Transcriptome Analysis of Tetraploid Cells Identifies Cyclin D2 as a Facilitator of Adaptation to Genome Doubling in the Presence of P53. Mol. Biol. Cell 2016, 27, 3065–3084.
- Katsuda, T.; Hosaka, K.; Matsuzaki, J.; Usuba, W.; Prieto-Vila, M.; Yamaguchi, T.; Tsuchiya, A.; Terai, S.; Ochiya, T. Transcriptomic Dissection of Hepatocyte Heterogeneity: Linking Ploidy, Zonation, and Stem/Progenitor Cell Characteristics. Cell. Mol. Gastroenterol. Hepatol. 2020, 9, 161–183.
- Anatskaya, O.V.; Vinogradov, A.E.; Vainshelbaum, N.M.; Giuliani, A.; Erenpreisa, J. Phylostratic Shift of Whole-Genome Duplications in Normal Mammalian Tissues towards Unicellularity Is Driven by Developmental Bivalent Genes and Reveals a Link to Cancer. Int. J. Mol. Sci. 2020, 21, 8759.
- Pienta, K.J.; Hammarlund, E.U.; Brown, J.S.; Amend, S.R.; Axelrod, R.M. Cancer Recurrence and Lethality Are Enabled by Enhanced Survival and Reversible Cell Cycle Arrest of Polyaneuploid Cells. Proc. Natl. Acad. Sci. USA 2021, 118, e2020838118.
- Lazzeri, E.; Angelotti, M.L.; Conte, C.; Anders, H.-J.; Romagnani, P. Surviving Acute Organ Failure: Cell Polyploidization and Progenitor Proliferation. Trends Mol. Med. 2019, 25, 366–381.
- Clay, D.E.; Fox, D.T. DNA Damage Responses during the Cell Cycle: Insights from Model Organisms and Beyond. Genes 2021, 12, 1882.
- Øvrebø, J.I.; Edgar, B.A. Polyploidy in Tissue Homeostasis and Regeneration. Dev. Camb. Engl. 2018, 145, dev156034.
- Sikora, E.; Czarnecka-Herok, J.; Bojko, A.; Sunderland, P. Therapy-Induced Polyploidization and Senescence: Coincidence or Interconnection? Semin. Cancer Biol. 2020.
- Walen, K.H. Cell Cycle Stress in Normal Human Cells: A Route to “First Cells” (with/without Fitness Gain) and Cancer-like Cell-Shape Changes. In Seminars in Cancer Biology; Academic Press: Cambridge, MA, USA, 2021.
- Gjelsvik, K.J.; Besen-McNally, R.; Losick, V.P. Solving the Polyploid Mystery in Health and Disease. Trends Genet. 2019, 35, 6–14.
- Vazquez-Martin, A.; Anatskaya, O.V.; Giuliani, A.; Erenpreisa, J.; Huang, S.; Salmina, K.; Inashkina, I.; Huna, A.; Nikolsky, N.N.; Vinogradov, A.E. Somatic Polyploidy Is Associated with the Upregulation of C-MYC Interacting Genes and EMT-like Signature. Oncotarget 2016, 7, 75235–75260.
- Bailey, E.C.; Kobielski, S.; Park, J.; Losick, V.P. Polyploidy in Tissue Repair and Regeneration. Cold Spring Harb. Perspect. Biol. 2021, 13, a040881.
- Derks, W.; Bergmann, O. Polyploidy in Cardiomyocytes: Roadblock to Heart Regeneration? Circ. Res. 2020, 126, 552–565.
- Broughton, K.M.; Khieu, T.; Nguyen, N.; Rosa, M.; Mohsin, S.; Quijada, P.; Wang, B.J.; Echeagaray, O.H.; Kubli, D.A.; Kim, T.; et al. Cardiac Interstitial Tetraploid Cells Can Escape Replicative Senescence in Rodents but Not Large Mammals. Commun. Biol. 2019, 2, 205.
- Anatskaya, O.V.; Sidorenko, N.V.; Vinogradov, A.E.; Beyer, T.V. Impact of Neonatal Cryptosporidial Gastroenteritis on Epigenetic Programming of Rat Hepatocytes. Cell Biol. Int. 2007, 31, 420–427.
- Malik, A.; Korol, A.; Weber, M.; Hankeln, T.; Avivi, A.; Band, M. Transcriptome Analysis of the Spalax Hypoxia Survival Response Includes Suppression of Apoptosis and Tight Control of Angiogenesis. BMC Genom. 2012, 13, 615.
- Ma, S.; Gladyshev, V.N. Molecular Signatures of Longevity: Insights from Cross-Species Comparative Studies. Semin. Cell Dev. Biol. 2017, 70, 190–203.
- Van de Peer, Y.; Mizrachi, E.; Marchal, K. The Evolutionary Significance of Polyploidy. Nat. Rev. Genet. 2017, 18, 411–424.
- Mayfield-Jones, D.; Washburn, J.D.; Arias, T.; Edger, P.P.; Pires, J.C.; Conant, G.C. Watching the Grin Fade: Tracing the Effects of Polyploidy on Different Evolutionary Time Scales. Semin. Cell Dev. Biol. 2013, 24, 320–331.
- Michiue, T.; Yamamoto, T.; Yasuoka, Y.; Goto, T.; Ikeda, T.; Nagura, K.; Nakayama, T.; Taira, M.; Kinoshita, T. High Variability of Expression Profiles of Homeologous Genes for Wnt, Hh, Notch, and Hippo Signaling Pathways in Xenopus Laevis. Dev. Biol. 2017, 426, 270–290.
- Blanc, G.; Wolfe, K.H. Widespread Paleopolyploidy in Model Plant Species Inferred from Age Distributions of Duplicate Genes. Plant Cell 2004, 16, 1667–1678.
- Kind, J.; Pagie, L.; de Vries, S.S.; Nahidiazar, L.; Dey, S.S.; Bienko, M.; Zhan, Y.; Lajoie, B.; de Graaf, C.A.; Amendola, M.; et al. Genome-Wide Maps of Nuclear Lamina Interactions in Single Human Cells. Cell 2015, 163, 134–147.
- Stevens, T.J.; Lando, D.; Basu, S.; Atkinson, L.P.; Cao, Y.; Lee, S.F.; Leeb, M.; Wohlfahrt, K.J.; Boucher, W.; O’Shaughnessy-Kirwan, A.; et al. 3D Structures of Individual Mammalian Genomes Studied by Single-Cell Hi-C. Nature 2017, 544, 59–64.
- Malashicheva, A.; Perepelina, K. Diversity of Nuclear Lamin A/C Action as a Key to Tissue-Specific Regulation of Cellular Identity in Health and Disease. Front. Cell Dev. Biol. 2021, 9, 761469.
- Garcia-Lozano, M.; Natarajan, P.; Levi, A.; Katam, R.; Lopez-Ortiz, C.; Nimmakayala, P.; Reddy, U.K. Altered Chromatin Conformation and Transcriptional Regulation in Watermelon Following Genome Doubling. Plant J. Cell Mol. Biol. 2021, 106, 588–600.
- Kuga, T.; Nie, H.; Kazami, T.; Satoh, M.; Matsushita, K.; Nomura, F.; Maeshima, K.; Nakayama, Y.; Tomonaga, T. Lamin B2 Prevents Chromosome Instability by Ensuring Proper Mitotic Chromosome Segregation. Oncogenesis 2014, 3, e94.
- Han, L.; Choudhury, S.; Mich-Basso, J.D.; Ammanamanchi, N.; Ganapathy, B.; Suresh, S.; Khaladkar, M.; Singh, J.; Maehr, R.; Zuppo, D.A.; et al. Lamin B2 Levels Regulate Polyploidization of Cardiomyocyte Nuclei and Myocardial Regeneration. Dev. Cell 2020, 53, 42–59.e11.
- Silva, I.S.; Ghiraldini, F.G.; Veronezi, G.M.B.; Mello, M.L.S. Polyploidy and Nuclear Phenotype Characteristics of Cardiomyocytes from Diabetic Adult and Normoglycemic Aged Mice. Acta Histochem. 2018, 120, 84–94.
- Ghiraldini, F.G.; Silva, I.S.; Mello, M.L.S. Polyploidy and Chromatin Remodeling in Hepatocytes from Insulin-Dependent Diabetic and Normoglycemic Aged Mice. Cytom. Part J. Int. Soc. Anal. Cytol. 2012, 81, 755–764.
- Donne, R.; Saroul-Aïnama, M.; Cordier, P.; Celton-Morizur, S.; Desdouets, C. Polyploidy in Liver Development, Homeostasis and Disease. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 391–405.
- Zybina, T.G.; Zybina, E.V. Role of Cell Cycling and Polyploidy in Placental Trophoblast of Different Mammalian Species. Reprod. Domest. Anim. Zuchthyg. 2020, 55, 895–904.
- Gilsbach, R.; Preissl, S.; Grüning, B.A.; Schnick, T.; Burger, L.; Benes, V.; Würch, A.; Bönisch, U.; Günther, S.; Backofen, R.; et al. Dynamic DNA Methylation Orchestrates Cardiomyocyte Development, Maturation and Disease. Nat. Commun. 2014, 5, 5288.
- Zhang, J.; Liu, Y.; Xia, E.-H.; Yao, Q.-Y.; Liu, X.-D.; Gao, L.-Z. Autotetraploid Rice Methylome Analysis Reveals Methylation Variation of Transposable Elements and Their Effects on Gene Expression. Proc. Natl. Acad. Sci. USA 2015, 112, E7022–E7029.
- Bateson, P. Robustness and Plasticity in Development. Wiley Interdiscip. Rev. Cogn. Sci. 2017, 8, e1386.
- Gluckman, P.D.; Hanson, M.A.; Low, F.M. Evolutionary and Developmental Mismatches Are Consequences of Adaptive Developmental Plasticity in Humans and Have Implications for Later Disease Risk. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2019, 374, 20180109.
- Desplats, P.; Gutierrez, A.M.; Antonelli, M.C.; Frasch, M.G. Microglial Memory of Early Life Stress and Inflammation: Susceptibility to Neurodegeneration in Adulthood. Neurosci. Biobehav. Rev. 2020, 117, 232–242.
- Abbasi, Z.; Ghahramani Seno, M.M.; Fereidoni, M. A Neonatal Mild Defect in Brain Insulin Signaling Predisposes a Subclinical Model of Sporadic Alzheimer’s to Develop the Disease. J. Mol. Neurosci. MN 2021, 71, 1473–1484.
- Barker, D.J.; Osmond, C. Infant Mortality, Childhood Nutrition, and Ischaemic Heart Disease in England and Wales. Lancet Lond. Engl. 1986, 1, 1077–1081.
- Barker, D.J.P. Coronary Heart Disease: A Disorder of Growth. Horm. Res. 2003, 59 (Suppl. 1), 35–41.
- Golubnitschaja, O.; Costigliola, V. Predictive, Preventive and Personalised Medicine as the Medicine of the Future: Anticipatory Scientific Innovation and Advanced Medical Services. In Anticipation and Medicine; Nadin, M., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 69–85. ISBN 978-3-319-45140-4.
- Aagaard-Tillery, K.M.; Grove, K.; Bishop, J.; Ke, X.; Fu, Q.; McKnight, R.; Lane, R.H. Developmental Origins of Disease and Determinants of Chromatin Structure: Maternal Diet Modifies the Primate Fetal Epigenome. J. Mol. Endocrinol. 2008, 41, 91–102.
- Simmons, R.A. Developmental Origins of Adult Disease. Pediatr. Clin. N. Am. 2009, 56, 449–466.
- Goyal, D.; Limesand, S.W.; Goyal, R. Epigenetic Responses and the Developmental Origins of Health and Disease. J. Endocrinol. 2019, 242, T105–T119.
- Ryznar, R.J.; Phibbs, L.; Van Winkle, L.J. Epigenetic Modifications at the Center of the Barker Hypothesis and Their Transgenerational Implications. Int. J. Environ. Res. Public. Health 2021, 18, 12728.
- Lurbe, E.; Ingelfinger, J. Developmental and Early Life Origins of Cardiometabolic Risk Factors: Novel Findings and Implications. Hypertension 2021, 77, 308–318.
- Grilo, L.F.; Tocantins, C.; Diniz, M.S.; Gomes, R.M.; Oliveira, P.J.; Matafome, P.; Pereira, S.P. Metabolic Disease Programming: From Mitochondria to Epigenetics, Glucocorticoid Signalling and Beyond. Eur. J. Clin. Investig. 2021, 51, e13625.
- Hochberg, Z.; Feil, R.; Constancia, M.; Fraga, M.; Junien, C.; Carel, J.-C.; Boileau, P.; Le Bouc, Y.; Deal, C.L.; Lillycrop, K.; et al. Child Health, Developmental Plasticity, and Epigenetic Programming. Endocr. Rev. 2011, 32, 159–224.
- Fox, D.T.; Soltis, D.E.; Soltis, P.S.; Ashman, T.-L.; Van de Peer, Y. Polyploidy: A Biological Force From Cells to Ecosystems. Trends Cell Biol. 2020, 30, 688–694.
- Anatskaya, O.V.; Vinogradov, A.E. Heart and Liver as Developmental Bottlenecks of Mammal Design: Evidence from Cell Polyploidization. Biol. J. Linn. Soc. 2004, 83, 175–186.
- Anatskaya, O.V.; Vinogradov, A.E. Paradoxical Relationship between Protein Content and Nucleolar Activity in Mammalian Cardiomyocytes. Genome 2004, 47, 565–578.
- Brodsky, V.Y.; Sarkisov, D.S.; Arefyeva, A.M.; Panova, N.W.; Gvasava, I.G. Polyploidy in Cardiac Myocytes of Normal and Hypertrophic Human Hearts; Range of Values. Virchows Arch. Int. J. Pathol. 1994, 424, 429–435.
- Mollova, M.; Bersell, K.; Walsh, S.; Savla, J.; Das, L.T.; Park, S.-Y.; Silberstein, L.E.; Dos Remedios, C.G.; Graham, D.; Colan, S.; et al. Cardiomyocyte Proliferation Contributes to Heart Growth in Young Humans. Proc. Natl. Acad. Sci. USA 2013, 110, 1446–1451.
- Anatskaya, O.V.; Sidorenko, N.V.; Beyer, T.V.; Vinogradov, A.E. Neonatal Cardiomyocyte Ploidy Reveals Critical Windows of Heart Development. Int. J. Cardiol. 2010, 141, 81–91.
- Bensley, J.G.; Stacy, V.K.; De Matteo, R.; Harding, R.; Black, M.J. Cardiac Remodelling as a Result of Pre-Term Birth: Implications for Future Cardiovascular Disease. Eur. Heart J. 2010, 31, 2058–2066.
- Anatskaya, O.V.; Vinogradov, A.E. Myocyte Ploidy in Heart Chambers of Birds with Different Locomotor Activity. J. Exp. Zool. 2002, 293, 427–441.
- Vinogradov, A.E.; Anatskaya, O.V.; Kudryavtsev, B.N. Relationship of Hepatocyte Ploidy Levels with Body Size and Growth Rate in Mammals. Genome 2001, 44, 350–360.
- Brodsky, W.Y.; Uryvaeva, I.V. Cell Polyploidy: Its Relation to Tissue Growth and Function. Int. Rev. Cytol. 1977, 50, 275–332.
- Anatskaya, O.V.; Erenpreisa, J.A.; Nikolsky, N.N.; Vinogradov, A.E. Pairwise Comparison of Mammalian Transcriptomes Associated with the Effect of Polyploidy on the Expression Activity of Developmental Gene Modules. Cell Tissue Biol. 2016, 10, 122–132.
- Kirillova, A.; Han, L.; Liu, H.; Kühn, B. Polyploid Cardiomyocytes: Implications for Heart Regeneration. Dev. Camb. Engl. 2021, 148, dev199401.
- Patterson, M.; Barske, L.; Van Handel, B.; Rau, C.D.; Gan, P.; Sharma, A.; Parikh, S.; Denholtz, M.; Huang, Y.; Yamaguchi, Y.; et al. Frequency of Mononuclear Diploid Cardiomyocytes Underlies Natural Variation in Heart Regeneration. Nat. Genet. 2017, 49, 1346–1353.
- Herget, G.W.; Neuburger, M.; Plagwitz, R.; Adler, C.P. DNA Content, Ploidy Level and Number of Nuclei in the Human Heart after Myocardial Infarction. Cardiovasc. Res. 1997, 36, 45–51.
- Gan, P.; Patterson, M.; Velasquez, A.; Wang, K.; Tian, D.; Windle, J.J.; Tao, G.; Judge, D.P.; Makita, T.; Park, T.J.; et al. Tnni3k Alleles Influence Ventricular Mononuclear Diploid Cardiomyocyte Frequency. PLoS Genet. 2019, 15, e1008354.
- Bergmann, O. Cardiomyocytes in Congenital Heart Disease: Overcoming Cytokinesis Failure in Tetralogy of Fallot. J. Thorac. Cardiovasc. Surg. 2021, 161, 1587–1590.
- Anatskaya, O.V.; Matveev, I.V.; Sidorenko, N.V.; Kharchenko, M.V.; Kropotov, A.V.; Vinogradov, A.E. Changes in the Heart of Neonatal Rats after Cryptosporidial Gastroenteritis of Different Degrees of Severity. J. Evol. Biochem. Physiol. 2013, 49, 509–518.
- Finch, C.E. Evolution in Health and Medicine Sackler Colloquium: Evolution of the Human Lifespan and Diseases of Aging: Roles of Infection, Inflammation, and Nutrition. Proc. Natl. Acad. Sci. USA 2010, 107 (Suppl. 1), 1718–1724.
- Curione, M.; Aratari, A.; Amato, S.; Colotto, M.; Barbato, M.; Leone, S.; Tego, A.; Panetti, D.; Parlapiano, C. A Study on QT Interval in Patients Affected with Inflammatory Bowel Disease without Cardiac Involvement. Intern. Emerg. Med. 2010, 5, 307–310.
- Filatova, N.A.; Knyazev, N.A.; Skarlato, S.O.; Anatskaya, O.V.; Vinogradov, A.E. Natural Killer Cell Activity Irreversibly Decreases after Cryptosporidium Gastroenteritis in Neonatal Mice. Parasite Immunol. 2018, 40, e12524.
- Anatskaya, O.V.; Erenpreisa, J.A.; Salmina, K.A.; Vazquez-Martin, A.; Huna, A.; Nikolsky, N.N.; Vinogradov, A.E. Polyploidy Activates Biological Pathways Related to Morphogenesis in Mammalian Tissues. MOJ Immunol. 2018, 6, 90–93.
- Anatskaya, O.V.; Vinogradov, A.E. Somatic Polyploidy Promotes Cell Function under Stress and Energy Depletion: Evidence from Tissue-Specific Mammal Transcriptome. Funct. Integr. Genom. 2010, 10, 433–446.
- Kimmel, G.J.; Dane, M.; Heiser, L.M.; Altrock, P.M.; Andor, N. Integrating Mathematical Modeling with High-Throughput Imaging Explains How Polyploid Populations Behave in Nutrient-Sparse Environments. Cancer Res. 2020, 80, 5109–5120.
- Anatskaya, O.V.; Matveev, I.V.; Sidorenko, N.V.; Kharchenko, M.V.; Kropotov, A.V.; Vinogradov, A.E. Remodeling of Rat Cardiomyocytes after Neonatal Cryptosporidiosis. I. Change of Ratio of Isoforms of Myosin Heavy Chains. Cell Tissue Biol. 2012, 6, 40–51.
- Han, P.; Li, W.; Yang, J.; Shang, C.; Lin, C.-H.; Cheng, W.; Hang, C.T.; Cheng, H.-L.; Chen, C.-H.; Wong, J.; et al. Epigenetic Response to Environmental Stress: Assembly of BRG1-G9a/GLP-DNMT3 Repressive Chromatin Complex on Myh6 Promoter in Pathologically Stressed Hearts. Biochim. Biophys. Acta 2016, 1863, 1772–1781.
- Petruseva, I.O.; Evdokimov, A.N.; Lavrik, O.I. Genome Stability Maintenance in Naked Mole-Rat. Acta Nat. 2017, 9, 31–41.
- Lucchetta, E.M.; Ohlstein, B. Amitosis of Polyploid Cells Regenerates Functional Stem Cells in the Drosophila Intestine. Cell Stem Cell 2017, 20, 609–620.e6.
- Alié, A.; Hayashi, T.; Sugimura, I.; Manuel, M.; Sugano, W.; Mano, A.; Satoh, N.; Agata, K.; Funayama, N. The Ancestral Gene Repertoire of Animal Stem Cells. Proc. Natl. Acad. Sci. USA 2015, 112, E7093–E7100.
- Ruiz-Trillo, I.; de Mendoza, A. Towards Understanding the Origin of Animal Development. Dev. Camb. Engl. 2020, 147, dev192575.
- Vinogradov, A.E. Accelerated Pathway Evolution in Mouse-like Rodents Involves Cell Cycle Control. Mamm. Genome 2015, 26, 609–618.
- Vinogradov, A.E.; Anatskaya, O.V. Gene Golden Age Paradox and Its Partial Solution. Genomics 2019, 111, 115–126.
- Sikora, E.; Bielak-Zmijewska, A.; Mosieniak, G. Cellular Senescence in Ageing, Age-Related Disease and Longevity. Curr. Vasc. Pharmacol. 2014, 12, 698–706.
