The primary cilium is a small microtubule-based organelle that extends from the apical surface of most eukaryotic cells into the extracellular space for sensing and transducing a wide range of cues. Defects in cilia growth and function are associated with a group of human inherited multisystemic diseases, known as ciliopathies. In recent years a rising number of ciliary proteins have been described at extraciliary sites, both in ciliated and non-ciliated cells, and have been implicated in cilium-independent functions and different cellular processes. Hematopoietic cells, including T lymphocytes, do not form primary cilia. However, non-ciliated T cells co-opt the ciliogenesis machinery for the assembly and function of the immunological synapse, a well-organized structure formed by immune cells – multiple types of T cells, mast cells, NK cells, B cells, neutrophils, macrophages, and dendritic cells – allowing for antigen recognition and signaling, information exchange and polarized release of molecules into the synaptic cleft. The identification of many, unexpected similarities between the primary cilium and the T cell immunological synapse at the structural, functional and molecular levels have highlighted the homology between these structures, even though they show disparate morphologies.
- primary cilium
- immunological synapse
- homology
1. An overview of the immunological synapse and the primary cilium
The immunological synapse (IS) is a specialized, adhesive junction that forms between either immune cells (e.g. a T helper cell and a B cell), or an immune cell and a different cell type (e.g. a cytotoxic T cell and its cognate target). This structure is characterized by: (i) close apposition of cell membranes that seals off a nanoscale space, called the synaptic cleft; (ii) cell-cell adhesion mediated by integrins; (iii) junction stability relative to the highly dynamic behavior of T lymphocytes in secondary lymphoid tissues; (iv) directed secretion of soluble factors (i.e. cytokines and cytotoxic molecules) in response to TCR signaling[1]. In the 1970s puzzling evidence highlighted physical interactions between immune cells as required for T cell help and cytotoxicity[2] and the relevance of this interaction to T cell activation and function was synthetized by Norcross as “synaptic basic for T cell activation”[3]. However, only in 1998 Kupfer took advantage of the rapid advances in fluorescence microscopy and molecular immunology to carry out a 3D analysis of T cell-APC conjugates, revealing the spatial organization of receptors and adhesion molecules in distinct SupraMolecular Activation Clusters within the IS. The mature bull’s eye synapse consists of three concentric regions[4]: a central SMAC (cSMAC) enriched in clustered T cell antigen receptors (TCRs) and the co-stimulatory receptor CD28, a peripheral SMAC (pSMAC) where adhesion molecules, such as LFA-1, are accumulated allowing for a tight adhesion between the cells and a lamellipodium-like distal SMAC (dSMAC).
The primary cilium is a single, non-motile projection that emerges from the surface of most cell types with some variations depending on cell cycle and differentiation stages[5]. First observed in the 19th century by Zimmermann[6], the primary cilium has long been considered an organelle that had lost its mobility function, and therefore a rudimental organelle, due to its 9+0 axoneme lacking the central microtubule pair and dynein arms. The fact that mutations in genes encoding for primary cilium components are cause of a wide range of human diseases, collectively known as ciliopathies[7], has led to rethink the primary cilium function. An in-depth study of ciliopathy-associated phenotypes has highlighted the signaling function of the primary cilium as well as the mechanisms and the regulators that control its assembly and maintenance.
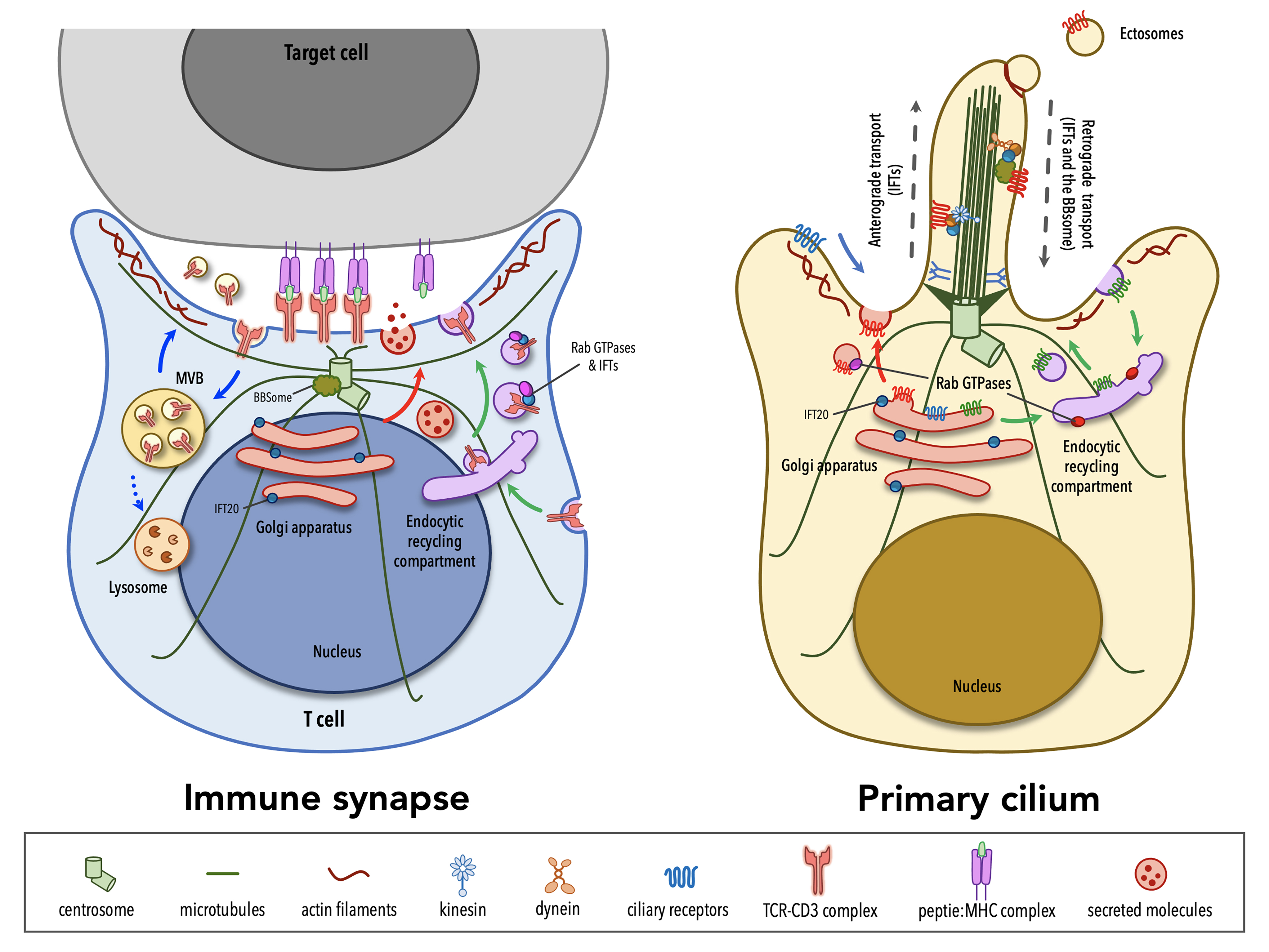
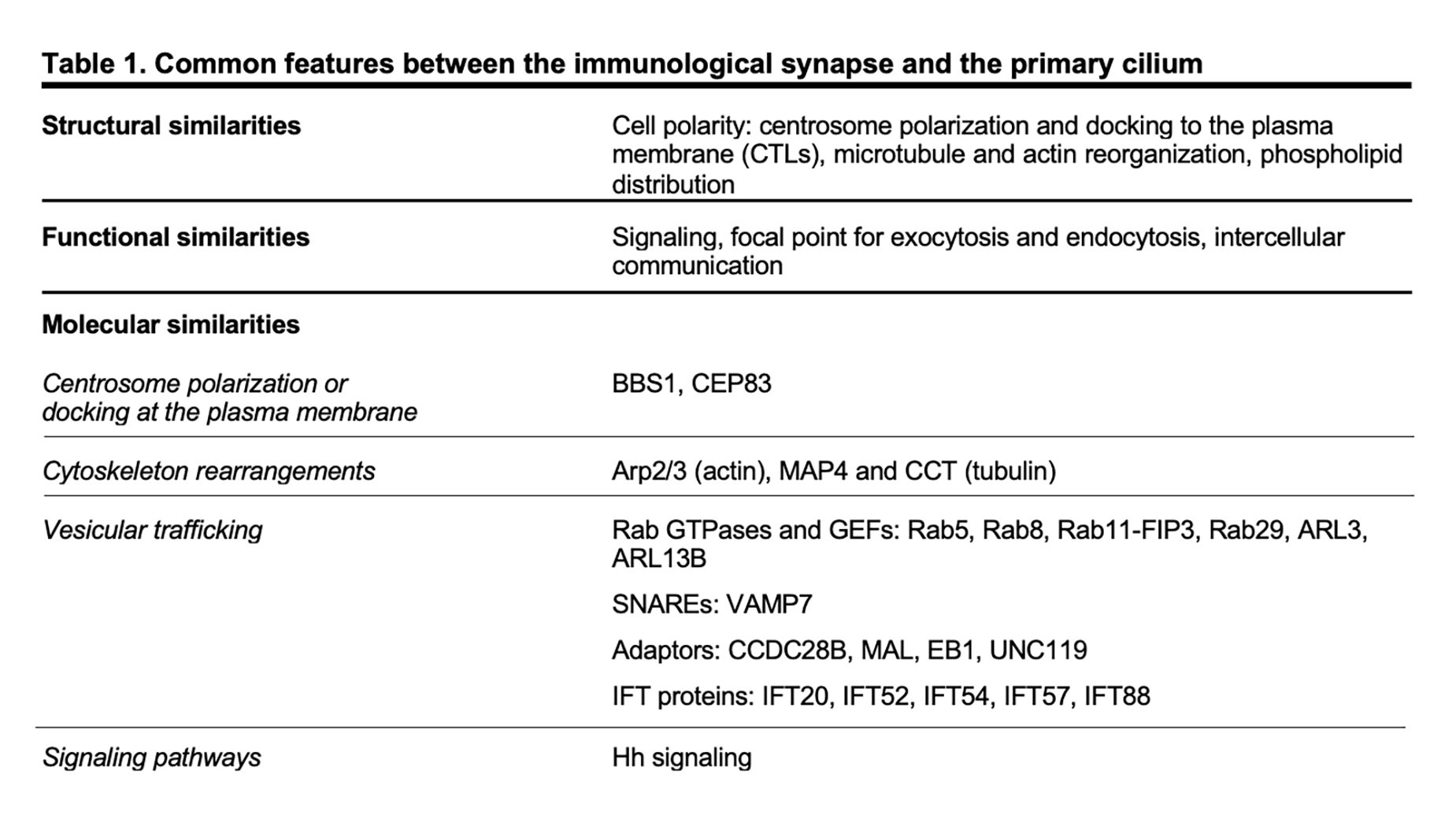
Although lymphocytes are one of the few cell types that lack primary cilia[8][9], electron micrographs of the IS showed that the centrosome docks at the synaptic membrane in CTLs, which is similar to the positioning of the basal body in the primary cilium, suggesting that these cells are able to form a “frustrated cilium” with centrosome docking at the synaptic membrane but not cilium elongation[10][11]. Additionally, T lymphocytes were found to express Intraflagellar Transport (IFT) proteins[12], which are essential for cilium formation and function, and to exploit them for vesicular trafficking and IS assembly. In subsequent years, several structural, functional and molecular parallels have revealed striking similarities between the IS and the primary cilium (Table 1), leading to the hypothesis that these structures might be functional homologues.
2. Structural Similarities
The primary cilium and the IS are prime examples of polarized structures, the formation of which is concomitant with an asymmetric re-organization of cytoskeleton, cytoplasmic and plasma membrane-associated molecules, as well as intracellular organelles.
A hallmark of cell polarity is the reorientation of the centrosome, the most common microtubule-organizing center (MTOC)[13], that moves towards the apical side of the cell and eventually establishes a physical contact with the plasma membrane. The centrosome consists of two centrioles, the mother centriole endowed of distal and subdistal appendages and the daughter centriole lacking appendages, surrounded by a pericentrosomal material (PCM)[14]. An early event in ciliogenesis is the centrosome-to-basal-body conversion[15], a process that in some contexts relies on vesicular trafficking. Indeed, vesicles originating at the Golgi complex or at the recycling compartment accumulate on the top of the mother centriole to form a capping vesicle that moves in association with the mother centriole and fuses with the plasma membrane once it reaches the surface. The basal body docks via the distal appendages of the mother centriole to a portion of plasma membrane, which acquires some specialization features to become the ciliary membrane[16][17][18][19]. The polymerization of nine doublets of microtubules drives axoneme elongation, giving rise to a protrusion that emerges from the cell surface. In immune cells, the centrosome re-posits just beneath the synaptic membrane and only in the case of cytotoxic T cells (CTL) the mother centriole’s distal appendages have been observed to anchor the centrosome to the plasma membrane, allowing for the microtubule-mediated transport of lytic granules and their subsequent release to kill a target cell[10][11]. Centrosome polarization is mainly driven by cytosokeleton reorganization, even though a minor contribution of vesicular trafficking to this process cannot be ruled out as witnessed by the presence of a capping vesicle, which resembles that one of ciliated cells, over one of the centrioles in CD4+ T cells[20]. Of note, centrosome polarization does not only orient the microtubule array that originates from it, but also dictates the recruitment of other organelles, including the Golgi apparatus, and of vesicles that use microtubules as tracks for their directional, molecular-mediated movement towards the base of the primary cilium or in the IS proximity[21][22][10].
In addition to changes in the general microtubule organization, rearrangements of the actin cytoskeleton at the cell cortex occur during the early stages of ciliogenesis and IS assembly. A combination of proteomic and microscopy approaches has identified the presence of actin-binding proteins in the ciliary compartment[23][24][25][26][27][28], highlighting the importance of a more in-depth comprehension of actin cytoskeleton dynamics in the formation of primary cilia. Genetic depletion and inhibition studies, in which the turnover of actin filaments is tilted towards depolymerization, suggested that F-actin nucleation prevents cilia formation[29][30][31][32]. However, the role of the actin cytoskeleton in ciliogenesis is still controversial. In fact, more recent evidence has involved actin-binding proteins in ciliogenesis and cilia maintenance[33]. Remodeling of cortical actin is well described in T lymphocytes. The initial interaction between a T lymphocyte and its cognate cell results in the accumulation of actin at the contact site[34][35][36], followed by the reorganization of cortical actin in three discrete actin networks surrounding a central zone, which is relatively free of actin[37]: from the edge to the center, a lamellipodial branched actin network, a lamellar acto-myosin network, and actin foci that are thought to be associated with invadosome-like protrusions (ILPs). Beyond the dynamics of cortical actin, a common feature in ciliated and in non-ciliated cells is that the centrosome moves towards a hypodense actin region[38][11], where it has access to the plasma membrane for docking. Furthermore, an emerging role for a centrosomal pool of filamentous actin in ciliogenesis and IS assembly has been recently described. In ciliated cells, the transport of periciliary vesicles from the pericentrosomal region to the distal appendages of the mother centriole for primary cilium growth is mediated by the actin-based motor protein myosin-Va[39]. On the contrary, in non-ciliated T cells a local depletion of centrosomal F-actin is required for centrosome detachment from the nuclear envelope, allowing for its repositioning to the IS[40][41].
Consistent with their signaling function, both the IS and the primary cilium membrane show a specialized composition, enriched in receptors and signaling mediators, as well as in lipid rafts and phospholipids. Specifically, phospholipid distribution within the ciliary membrane is very similar to that one across the synapse, with an accumulation of phosphatidylinositol 4-phosphate PI(4)P in both ciliary and synaptic membrane that is replaced by phosphatidylinositol 4,5-biphosphate (PI(4,5)P2) and phosphatidylinositol 3,4,5-thipshosphate (PI(3,4,5)-P3) at the base of the cilium and at the edge of the IS[42][43][44]. A local accumulation of PI(4,5)P2, a lipid that is known to interact with actin-regulating proteins[45], at the tip of the primary cilium is a peculiarity of primary cilia, where both the release of ectosomes and the loss of the ciliary tips itself through an unusual form of cilia decapitation have been described as actin-mediated processes[46][47].
3. Functional Similarities
Both the primary cilium and the IS have been extensively characterized as signaling devices. Cells engaged in a tight junction for minutes to hours exchange information via chemical signals (i.e. membrane-associated receptors and soluble molecules) and physical forces that are generated via the spatiotemporal organization and dynamics of the cell cytoskeleton. Each cell integrates information coming from different signaling pathways and produces appropriate biochemical and genetic responses. Although historically regarded as a vestigial organelle, it has become clear that the primary cilium can sense a variety of extracellular stimuli and transducing these incoming signals into a cellular response with relevant outcomes in multiple cellular and developmental processes in vertebrates[48][49]. For this reason, the primary cilium is now referred to as “cell antenna”.
In addition to and in many instances related to signaling, both the IS and the primary cilium are vesicular trafficking hotspots. This is the consequence of the fact that, as the primary cilium and the IS assemble, the Golgi apparatus and the recycling/endosomal compartment polarize just beneath the base of the cilium and the synaptic membrane, respectively. At the base of the primary cilium the periciliary membrane invaginates to form a cytoplasmatic invagination called the ciliary pocket (CiPo), acting as a specialized site for localized endocytosis and exocytosis of vesicles that accompany the delivery and retrieval of ciliary components to and from the primary cilium[50][51]. Several studies have demonstrated that cilia growth and function require the directional delivery of vesicles carrying lipids and some ciliary membrane proteins from the Golgi or recycling endosomes to the periciliary membrane region, where vesicles undergo exocytosis and lipids are incorporated into the ciliary membrane[52]. Alternatively, some ciliary membrane proteins can enter the ciliary membrane from the plasma membrane through lateral diffusion[53][54][55][56]. Protein trafficking within the ciliary compartment is mediated by IFT particles, each comprising the IFT-A and IFT-B subcomplexes, which move along axonemal microtubules in association with the molecular motors kinesin-2 and cytoplasmic dynein-2[57][58]. The termination of many signaling pathways triggered at the primary cilium is regulated through the clearance of specific receptors from the ciliary compartment[59][60] and some of these are internalized at the ciliary pocket through clathrin-dependent endocytosis[48]. When the transport of activated ciliary receptors from cilia back into the cell fails, they can be incorporated into ectosomes that bud from the tip of the primary cilium with the assistance of newly polymerized actin filaments[46]. Some studies have demonstrated that the primary cilium is able not only to receive incoming signals, but also to produce signals that are transferred to other cells via extracellular vesicles (EVs)[61]. The functions of EVs released at the primary cilium are still poorly investigated. Recent evidence suggests the release of EVs as a means for a rapid modulation of the protein composition of the ciliary compartment, with relevant implications in signaling[46][62]. Although the role of ciliary EVs in intercellular communication is only starting to be experimentally tested, ciliary EVs have been also reported to be transferred from one organism to another and to influence mating behavior in Caenorhabditis elegans[63].
Similar to ciliogenesis, IS assembly and maintenance rely on the directional transport of endosomes containing receptors and signaling molecules that are known to exploit the recycling pathway to accumulate at the contact site with a cognate cell. Moreover, the IS serves as a focal point for both exocytosis and endocytosis that are triggered by TCR signaling at the plasma membrane[64]. The TCR itself constantly recycles from the cell surface to the recycling compartment and back to the plasma membrane as a mechanism of quality control of its signaling components[65][66]. However, upon engagement by a pMHC ligand, TCR surface levels are down-regulated as exhausted TCRs are internalized and sorted for recycling or lysosomal degradation[67]. Additionally, some of the post-endocytic TCRs together with a variety of cargoes, ranging from death receptor ligands, co-stimulatory receptors, microRNAs, genomic and mitochondrial DNA, are included in EVs that are focally released into the synaptic cleft, delivering signals to the target cell[68][69][70][71][72]. Polarized exocytosis is exploited also by activated T helper cells and CTLs for the focal release of vesicles containing cytokines or specialized secretory lysosomes carrying a battery of granzymes and the lytic pore-forming protein perforin[73][74].
4. Molecular Similarities
In recent years many of the proteins labelled as “ciliary proteins”, as they have been discovered and then characterized in the context of the primary cilium, have been shown to also localize outside the primary cilium and to perform extraciliary functions, suggesting that these proteins play a general role in a range of different cellular processes (i.e. cell cycle, cytoskeleton re-organization, vesicular trafficking) and that are, directly or indirectly, linked to cell polarity.[75][76] Among others, ciliary proteins have been implicated in different mechanisms of IS assembly, ranging from centrosome polarization and docking at the plasma membrane, to actin cytoskeleton rearrangements and vesicular trafficking.
The ciliopathy-related Bardet-Biedl syndrome 1 component, a core component of the BBS complex that is mainly involved in the retrieval of membrane proteins (e.g. G-protein-coupled receptors) from the primary cilium into the cell[77][78], also participates in CD4+ T cell IS assembly by regulating the trafficking of proteasomal regulatory 19S subunit to the centrosome, where an increased activity of the local proteasome is required for centrosome polarization to the IS[41]. A peculiar feature of CTL IS is centrosome anchoring to the plasma membrane through the distal appendages of the mother centriole[11], allowing microtubules to re-organize just beneath the plasma membrane, thereby ensuring the directional transport of cytotoxic granules towards a specialized secretory domain within the synapse. The master distal appendage protein CEP83, which is required for ciliogenesis[79][80][81], shows a conserved function in non-ciliated CTLs, since its silencing by using RNA interference results in a reduced secretion of granules at the lytic synapse[11].
Consistent with the involvement of actin rearrangement in the assembly of both the primary cilium and the IS, the actin-related complex Arp2/3 is a shared participant in these processes. The Arp2/3 complex not only generates a branched actin network at the IS that facilitates T cell spreading[82][83] but also controls ciliogenesis and cilia length[29][31][39]. Furthermore, regulators of the tubulin cytoskeleton, such as the microtubule-associated protein 4 (MAP4) and the chaperonin containing T-complex protein (CCT, also known as TRiC) that controls cilia length and formation[84][85], have been later implicated in tubulin post-translational modifications and thus microtubule stability, on which centrosome polarization and vesicle dynamics depend[86][87].
Vesicular trafficking, and in particular the trafficking pathway responsible for TCR recycling to the IS, has been extensively investigated in recent years[88]. Surprisingly, some ciliogenesis-related proteins, which belong to the protein families of Rab GTPases together with their guanine nucleotide exchange factors (GEF)[89][90][91][92][93][94][95][96][97] and of soluble NSF attachment protein receptors (SNARE)[98], as well as some adaptor molecules[99][100][101][102] and IFT proteins[103], have now emerged as new players in IS assembly by controlling the synaptic recruitment of the TCR itself and of other receptors and signaling molecules that are known to accumulate at the IS[12][66][97][104][105][106][107][108][109][110][111][112][113] (e.g. the transferrin receptor, the chemokine receptor CXCR4, the transmembrane adaptor LAT, the kinase LCK). Most ciliary proteins specifically implicated in IS assembly have been mapped on the two main arms of the recycling pathway, regulated by such as Rab4 and Rab11, suggesting the existence of different subpopulations of recycling endosomes that, while participating in the canonical recycling pathways orchestrated by Rab4 and Rab11, are responsible for carrying specific cargo destined for the synaptic membrane.
The primary cilium is essential for the transduction of Hedgehog (Hh) signaling[114][115]. Binding of exogenous ligands (i.e. Sonic, Indian or Desert Hh) to the trans-membrane receptor Patched allows the ciliary translocation and activation of the signal transducer Smoothened and the subsequent activation of Gli and Gli-mediated transcription of target genes. Following TCR activation, CTLs express Hh pathway components, including Hh ligands, Patched, Smoothened and Gli. In the non-ciliated CTLs, Hh signaling promotes a Rac1-dependent remodeling of cortical actin, which is instrumental for centrosome polarization and CTL-mediated killing[116].
This entry is adapted from the peer-reviewed paper 10.3390/cells8080789
References
- Michael L. Dustin; Modular Design of Immunological Synapses and Kinapses. Cold Spring Harbor Perspectives in Biology 2009, 1, a002873-a002873, 10.1101/cshperspect.a002873.
- Martin C. Raff; T and B Lymphocytes and Immune Responses. Nature 1973, 242, 19-23, 10.1038/242019a0.
- M.A. Norcross; A synaptic basis for T-lymphocyte activation. Annales de l'Institut Pasteur / Immunologie 1984, 135, 113-134, 10.1016/s0769-2625(84)81105-8.
- Colin R. F. Monks; Benjamin A. Freiberg; Hannah Kupfer; Noah Sciaky; Abraham Kupfer; Three-dimensional segregation of supramolecular activation clusters in T cells. Nature 1998, 395, 82-86, 10.1038/25764.
- D N Wheatley; EXPRESSION OF PRIMARY CILIA IN MAMMALIAN CELLS. Cell Biology International 1996, 20, 73-81, 10.1006/cbir.1996.0011.
- K. W. Zimmermann; Beiträge zur Kenntniss einiger Drüsen und Epithelien. Archiv für Mikroskopische Anatomie 1898, 52, 552-706, 10.1007/bf02975837.
- Jeremy F. Reiter; Michel R. Leroux; Genes and molecular pathways underpinning ciliopathies. Nature Reviews Molecular Cell Biology 2017, 18, 533-547, 10.1038/nrm.2017.60.
- Denys N. Wheatley; Primary Cilia in Normal and Pathological Tissues. Pathobiology 1995, 63, 222-238, 10.1159/000163955.
- Friedhelm Hildebrandt; Edgar Otto; Cilia and centrosomes: a unifying pathogenic concept for cystic kidney disease?. Nature Reviews Microbiology 2005, 6, 928-940, 10.1038/nrg1727.
- Jane C. Stinchcombe; Endre Majorovits; Giovanna Bossi; Stephen J Fuller; Gillian Griffiths; Centrosome polarization delivers secretory granules to the immunological synapse. Nature 2006, 443, 462-465, 10.1038/nature05071.
- Jane C. Stinchcombe; Lyra O. Randzavola; Karen Angus-Cole; Judith M. Mantell; Paul Verkade; Gillian M. Griffiths; Mother Centriole Distal Appendages Mediate Centrosome Docking at the Immunological Synapse and Reveal Mechanistic Parallels with Ciliogenesis. Current Biology 2015, 25, 3239-3244, 10.1016/j.cub.2015.10.028.
- Francesca Finetti; Silvia Rossi Paccani; Maria Giovanna Riparbelli; Emiliana Giacomello; Giuseppe Perinetti; Gregory Pazour; Joel L. Rosenbaum; Cosima Baldari; Intraflagellar transport is required for polarized recycling of the TCR/CD3 complex to the immune synapse. Nature Cell Biology 2009, 11, 1332-1339, 10.1038/ncb1977.
- D R Kellogg; M Moritz; B M Alberts; The Centrosome and Cellular Organization. Annual Review of Biochemistry 1994, 63, 639-674, 10.1146/annurev.biochem.63.1.639.
- Swadhin Chandra Jana; Centrosome structure and biogenesis: Variations on a theme?. Seminars in Cell & Developmental Biology 2021, 110, 123-138, 10.1016/j.semcdb.2020.10.014.
- S P Sorokin; Centriole formation and ciliogenesis.. Aspen Emphysema Conference 1968, 11, 213-6, .
- Jeremy F Reiter; Oliver E Blacque; Michel R Leroux; The base of the cilium: roles for transition fibres and the transition zone in ciliary formation, maintenance and compartmentalization. EMBO reports 2012, 13, 608-618, 10.1038/embor.2012.73.
- Quanlong Lü; Christine Insinna; Carolyn Ott; Jimmy Stauffer; Petra Alexandra Rodrigues Pintado; Juliati Rahajeng; Ulrich Baxa; Vijay Walia; Adrian Cuenca; Yoo-Seok Hwang; et al. Early steps in primary cilium assembly require EHD1/EHD3-dependent ciliary vesicle formation. Nature Cell Biology 2015, 17, 228-240, 10.1038/ncb3109.
- Laura E. Yee; Jeremy F. Reiter; Ciliary Vesicle Formation: A Prelude to Ciliogenesis. Developmental Cell 2015, 32, 665-666, 10.1016/j.devcel.2015.03.012.
- Lei Wang; Brian D. Dynlacht; The regulation of cilium assembly and disassembly in development and disease. Development 2018, 145, dev151407, 10.1242/dev.151407.
- Andres Ernesto Zucchetti; Laurence Ardouin-Bataille; Jean-Marie Carpier; Stéphanie Dogniaux; Mabel San Roman-Jouve; Mathieu Maurin; Michael W. Stuck; Rosa M. Rios; Cosima Baldari; Gregory J. Pazour; et al. Tethering of vesicles to the Golgi by GMAP210 controls LAT delivery to the immune synapse. Nature Communications 2019, 10, 1-17, 10.1038/s41467-019-10891-w.
- A Kupfer; G Dennert; S J Singer; Polarization of the Golgi apparatus and the microtubule-organizing center within cloned natural killer cells bound to their targets.. Proceedings of the National Academy of Sciences 1983, 80, 7224-7228, 10.1073/pnas.80.23.7224.
- C. Anthony Poole; Michael H. Flint; Brent W. Beaumont; Analysis of the morphology and function of primary cilia in connective tissues:A cellular cybernetic probe?. Cell Motility 1985, 5, 175-193, 10.1002/cm.970050302.
- Michael H. Chaitin; Richard B. Carlsen; Ghassan J. Samara; Immunogold localization of actin in developing photoreceptor cilia of normal and rds mutant mice. Experimental Eye Research 1988, 47, 437-446, 10.1016/0014-4835(88)90054-1.
- Qin Liu; Glenn Tan; Natasha Levenkova; Tiansen Li; Edward N. Pugh; John J. Rux; David W. Speicher; Eric A. Pierce; The Proteome of the Mouse Photoreceptor Sensory Cilium Complex. Molecular & Cellular Proteomics 2007, 6, 1299-1317, 10.1074/mcp.m700054-mcp200.
- Hiroaki Ishikawa; James Thompson; John R. Yates; Wallace F. Marshall; Proteomic Analysis of Mammalian Primary Cilia. Current Biology 2012, 22, 414-419, 10.1016/j.cub.2012.01.031.
- Gagan D. Gupta; Etienne Coyaud; João Gonçalves; Bahareh A. Mojarad; Yi Liu; Qianzhu Wu; Ladan Gheiratmand; David Comartin; Johnny M. Tkach; Sally W.T. Cheung; et al. A Dynamic Protein Interaction Landscape of the Human Centrosome-Cilium Interface. Cell 2015, 163, 1484-1499, 10.1016/j.cell.2015.10.065.
- Priyanka Kohli; Martin Höhne; Christian Jüngst; Sabine Bertsch; Lena K Ebert; Astrid C Schauss; Thomas Benzing; Markus M Rinschen; Bernhard Schermer; The ciliary membrane‐associated proteome reveals actin‐binding proteins as key components of cilia. EMBO reports 2017, 18, 1521-1535, 10.15252/embr.201643846.
- Petra Kiesel; Gonzalo Alvarez Viar; Nikolai Tsoy; Riccardo Maraspini; Peter Gorilak; Vladimir Varga; Alf Honigmann; Gaia Pigino; The molecular structure of mammalian primary cilia revealed by cryo-electron tomography. Nature Structural & Molecular Biology 2020, 27, 1115-1124, 10.1038/s41594-020-0507-4.
- Joon Kim; Ji Eun Lee; Susanne Heynen-Genel; Eigo Suyama; Keiichiro Ono; Kiyoung Lee; Trey Ideker; Pedro Aza-Blanc; Joseph G. Gleeson; Functional genomic screen for modulators of ciliogenesis and cilium length. Nature 2010, 464, 1048-1051, 10.1038/nature08895.
- Marina Bershteyn; Scott X. Atwood; Wei-Meng Woo; Mischa Li; Anthony E. Oro; MIM and Cortactin Antagonism Regulates Ciliogenesis and Hedgehog Signaling. Developmental Cell 2010, 19, 270-283, 10.1016/j.devcel.2010.07.009.
- Xiumin Yan; Xueliang Zhu; Branched F-actin as a negative regulator of cilia formation. Experimental Cell Research 2013, 319, 147-151, 10.1016/j.yexcr.2012.08.009.
- Jongshin Kim; Kim Jongshin; Hyowon Hong; Min Hwan Kim; Jin Man Kim; June-Koo Lee; Won Do Heo; Joon Kim; Actin remodelling factors control ciliogenesis by regulating YAP/TAZ activity and vesicle trafficking. Nature Communications 2015, 6, 6781-6781, 10.1038/ncomms7781.
- Claire E. L. Smith; Alice V. R. Lake; Colin A. Johnson; Primary Cilia, Ciliogenesis and the Actin Cytoskeleton: A Little Less Resorption, A Little More Actin Please. Frontiers in Cell and Developmental Biology 2020, 8, 622822, 10.3389/fcell.2020.622822.
- C J Sanderson; A M Glauert; The mechanism of T-cell mediated cytotoxicity. VI. T-cell projections and their role in target cell killing.. Immunology 1979, 36, 119-29, .
- Misty R Jenkins; Jane C Stinchcombe; Byron B Au-Yeung; Yukako Asano; Alex T Ritter; Arthur Weiss; Gillian M Griffiths; Distinct structural and catalytic roles for Zap70 in formation of the immunological synapse in CTL. eLife 2014, 3, e01310, 10.7554/elife.01310.
- Alex T. Ritter; Yukako Asano; Jane C. Stinchcombe; Nele Dieckmann; Bi-Chang Chen; Christian Gawden-Bone; Schuyler van Engelenburg; Wesley Legant; Liang Gao; Michael W. Davidson; et al. Actin Depletion Initiates Events Leading to Granule Secretion at the Immunological Synapse. Immunity 2015, 42, 864-876, 10.1016/j.immuni.2015.04.013.
- Daniel Blumenthal; Janis K. Burkhardt; Multiple actin networks coordinate mechanotransduction at the immunological synapse. The Journal of Cell Biology 2020, 219, e201911058, 10.1083/jcb.201911058.
- Stephen S. Francis; Jeff Sfakianos; Bryan Lo; Ira Mellman; A hierarchy of signals regulates entry of membrane proteins into the ciliary membrane domain in epithelial cells. Journal of Cell Biology 2011, 193, 219-233, 10.1083/jcb.201009001.
- Chien-Ting Wu; Hsin-Yi Chen; Tang K. Tang; Myosin-Va is required for preciliary vesicle transportation to the mother centriole during ciliogenesis. Nature Cell Biology 2018, 20, 175-185, 10.1038/s41556-017-0018-7.
- Ana Bello‐Gamboa; Marta Velasco; Solange Moreno; Gonzalo Herranz; Roxana Ilie; Silvia Huetos; Sergio Dávila; Alicia Sánchez; Jorge Bernardino De La Serna; Víctor Calvo; et al. Actin reorganization at the centrosomal area and the immune synapse regulates polarized secretory traffic of multivesicular bodies in T lymphocytes. Journal of Extracellular Vesicles 2020, 9, 1759926, 10.1080/20013078.2020.1759926.
- Chiara Cassioli; Anna Onnis; Francesca Finetti; Nagaja Capitani; Jlenia Brunetti; Ewoud B. Compeer; Veronika Niederlova; Ondrej Stepanek; Michael L. Dustin; Cosima T. Baldari; et al. The Bardet–Biedl syndrome complex component BBS1 controls T cell polarity during immune synapse assembly. Journal of Cell Science 2021, 134, jcs258462, 10.1242/jcs.258462.
- Marcelo Chávez; Sabrina Ena; Jacqueline Van Sande; Alban De Kerchove D'Exaerde; Stéphane Schurmans; Serge N. Schiffmann; Modulation of Ciliary Phosphoinositide Content Regulates Trafficking and Sonic Hedgehog Signaling Output. Developmental Cell 2015, 34, 338-350, 10.1016/j.devcel.2015.06.016.
- Francesc Garcia-Gonzalo; Siew Cheng Phua; Elle Roberson; Galo Garcia; Monika Abedin; Stéphane Schurmans; Takanari Inoue; Jeremy F. Reiter; Phosphoinositides Regulate Ciliary Protein Trafficking to Modulate Hedgehog Signaling. Developmental Cell 2015, 34, 400-409, 10.1016/j.devcel.2015.08.001.
- Christian Gawden-Bone; Gordon L. Frazer; Arianne C. Richard; Claire Ma; Katharina Strege; Gillian M. Griffiths; PIP5 Kinases Regulate Membrane Phosphoinositide and Actin Composition for Targeted Granule Secretion by Cytotoxic Lymphocytes. Immunity 2018, 49, 427-437.e4, 10.1016/j.immuni.2018.08.017.
- Rajat Rohatgi; Hsin-Yi Henry Ho; Marc W. Kirschner; Mechanism of N-Wasp Activation by Cdc42 and Phosphatidylinositol 4,5-Bisphosphate. Journal of Cell Biology 2000, 150, 1299-1310, 10.1083/jcb.150.6.1299.
- Andrew R. Nager; Jaclyn S. Goldstein; Vicente Herranz-Pérez; Didier Portran; Fan Ye; Jose Manuel Garcia-Verdugo; Maxence V. Nachury; An Actin Network Dispatches Ciliary GPCRs into Extracellular Vesicles to Modulate Signaling. Cell 2016, 168, 252-263.e14, 10.1016/j.cell.2016.11.036.
- Siew Cheng Phua; Shuhei Chiba; Masako Suzuki; Emily Su; Elle Roberson; Ganesh Pusapati; Mitsutoshi Setou; Rajat Rohatgi; Jeremy F. Reiter; Koji Ikegami; et al. Dynamic Remodeling of Membrane Composition Drives Cell Cycle through Primary Cilia Excision. Cell 2017, 168, 264-279.e15, 10.1016/j.cell.2016.12.032.
- Lotte B. Pedersen; Johanne B. Mogensen; Søren T. Christensen; Endocytic Control of Cellular Signaling at the Primary Cilium. Trends in Biochemical Sciences 2016, 41, 784-797, 10.1016/j.tibs.2016.06.002.
- Gabrielle Wheway; Liliya Nazlamova; John T. Hancock; Signaling through the Primary Cilium. Frontiers in Cell and Developmental Biology 2018, 6, 8, 10.3389/fcell.2018.00008.
- Rania Ghossoub; Anahi Molla-Herman; Philippe Bastin; Alexandre Benmerah; The ciliary pocket: a once-forgotten membrane domain at the base of cilia. Biology of the Cell 2011, 103, 131-144, 10.1042/bc20100128.
- Alexandre Benmerah; The ciliary pocket. Current Opinion in Cell Biology 2013, 25, 78-84, 10.1016/j.ceb.2012.10.011.
- Ching-Hwa Sung; Michel R. Leroux; The roles of evolutionarily conserved functional modules in cilia-related trafficking. Nature Cell Biology 2013, 15, 1387-1397, 10.1038/ncb2888.
- Ljiljana Milenkovic; Matthew P. Scott; Rajat Rohatgi; Lateral transport of Smoothened from the plasma membrane to the membrane of the cilium. Journal of Cell Biology 2009, 187, 365-374, 10.1083/jcb.200907126.
- Fan Ye; David K Breslow; Elena F Koslover; Andrew J Spakowitz; W James Nelson; Maxence V Nachury; Single molecule imaging reveals a major role for diffusion in the exploration of ciliary space by signaling receptors. eLife 2013, 2, e00654, 10.7554/elife.00654.
- Daisuke Takao; John F. Dishinger; H. Lynn Kee; Justine M. Pinskey; Ben L. Allen; Kristen J. Verhey; An Assay for Clogging the Ciliary Pore Complex Distinguishes Mechanisms of Cytosolic and Membrane Protein Entry. Current Biology 2014, 24, 2288-2294, 10.1016/j.cub.2014.08.012.
- Ljiljana Milenkovic; Lucien E. Weiss; Joshua Yoon; Theodore L. Roth; YouRong S. Su; Steffen J. Sahl; Matthew P. Scott; W. E. Moerner; Single-molecule imaging of Hedgehog pathway protein Smoothened in primary cilia reveals binding events regulated by Patched1. Proceedings of the National Academy of Sciences 2015, 112, 8320-8325, 10.1073/pnas.1510094112.
- Karl F. Lechtreck; IFT–Cargo Interactions and Protein Transport in Cilia. Trends in Biochemical Sciences 2015, 40, 765-778, 10.1016/j.tibs.2015.09.003.
- Ludek Stepanek; Gaia Pigino; Microtubule doublets are double-track railways for intraflagellar transport trains. Science 2016, 352, 721-724, 10.1126/science.aaf4594.
- Alexander Sorkin; Mark von Zastrow; Endocytosis and signalling: intertwining molecular networks. Nature Reviews Molecular Cell Biology 2009, 10, 609-622, 10.1038/nrm2748.
- Thomas E. Willnow; Annabel Christ; Annette Hammes; Endocytic receptor-mediated control of morphogen signaling. Development 2012, 139, 4311-4319, 10.1242/dev.084467.
- Juan Wang; Maureen M. Barr; Ciliary Extracellular Vesicles: Txt Msg Organelles. Cellular and Molecular Neurobiology 2016, 36, 449-457, 10.1007/s10571-016-0345-4.
- Ann-Kathrin Volz; Alina Frei; Viola Kretschmer; António M. De Jesus Domingues; Rene F. Ketting; Marius Ueffing; Karsten Boldt; Eva-Maria Krämer-Albers; Helen L. May-Simera; Bardet-Biedl syndrome proteins modulate the release of bioactive extracellular vesicles. Nature Communications 2021, 12, 1-16, 10.1038/s41467-021-25929-1.
- Juan Wang; Malan Silva; Leonard Haas; Natalia Morsci; Ken C.Q. Nguyen; David H. Hall; Maureen M. Barr; C. elegans Ciliated Sensory Neurons Release Extracellular Vesicles that Function in Animal Communication. Current Biology 2014, 24, 519-525, 10.1016/j.cub.2014.01.002.
- Gillian M. Griffiths; Andy Tsun; Jane C. Stinchcombe; The immunological synapse: a focal point for endocytosis and exocytosis. Journal of Cell Biology 2010, 189, 399-406, 10.1083/jcb.201002027.
- Carsten Geisler; TCR Trafficking in Resting and Stimulated T Cells. Critical Reviews in Immunology 2004, 24, 67-86, 10.1615/critrevimmunol.v24.30.
- Vincent Das; Beatrice Nal; Annick Dujeancourt; Maria-Isabel Thoulouze; Thierry Galli; Pascal Roux; Alice Dautry-Varsat; Andrés Alcover; Activation-Induced Polarized Recycling Targets T Cell Antigen Receptors to the Immunological Synapse: Involvement of SNARE Complexes. Immunity 2004, 20, 577-588, 10.1016/s1074-7613(04)00106-2.
- Haiyan Liu; Michele Rhodes; David L Wiest; Dario A.A Vignali; On the Dynamics of TCR:CD3 Complex Cell Surface Expression and Downmodulation. Immunity 2000, 13, 665-675, 10.1016/s1074-7613(00)00066-2.
- M J Martínez-Lorenzo; A Anel; S Gamen; I Monle N; P Lasierra; L Larrad; A Piñeiro; M A Alava; J Naval; Activated human T cells release bioactive Fas ligand and APO2 ligand in microvesicles.. The Journal of Immunology 1999, 163, 1274-81, .
- María Mittelbrunn; Cristina Gutierrez-Vazquez; Carolina Villarroya-Beltri; Susana González; Fatima Sanchez-Cabo; Manuel Ángel González; Antonio Bernad; Francisco Sánchez-Madrid; Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nature Communications 2011, 2, 282, 10.1038/ncomms1285.
- Kaushik Choudhuri; Jaime Llodrá; Eric W. Roth; Jones Tsai; Susana Gordo; Kai W. Wucherpfennig; Lance C. Kam; David L. Stokes; Michael L. Dustin; Polarized release of T-cell-receptor-enriched microvesicles at the immunological synapse. Nature 2014, 507, 118-123, 10.1038/nature12951.
- Daniel Torralba; Francesc Baixauli; Carolina Villarroya-Beltri; Irene Fernández Delgado; Ana Latorre-Pellicer; Rebeca Acin-Perez; Noa B Martín-Cófreces; Ángel Luis Jaso-Tamame; Salvador Iborra; Inmaculada Jorge; et al. Priming of dendritic cells by DNA-containing extracellular vesicles from activated T cells through antigen-driven contacts. Nature Communications 2018, 9, 1-17, 10.1038/s41467-018-05077-9.
- David G Saliba; Pablo F Céspedes-Donoso; Štefan Bálint; Ewoud B Compeer; Kseniya Korobchevskaya; Salvatore Valvo; Viveka Mayya; Audun Kvalvaag; Yanchun Peng; Tao Dong; et al. Composition and structure of synaptic ectosomes exporting antigen receptor linked to functional CD40 ligand from helper T cells. eLife 2019, 8, e47528, 10.7554/elife.47528.
- Morgan Huse; Björn F Lillemeier; Michael S Kuhns; Daniel Chen; Mark M Davis; T cells use two directionally distinct pathways for cytokine secretion. Nature Immunology 2006, 7, 247-255, 10.1038/ni1304.
- Jane C. Stinchcombe; Gillian M. Griffiths; Secretory Mechanisms in Cell-Mediated Cytotoxicity. Annual Review of Cell and Developmental Biology 2007, 23, 495-517, 10.1146/annurev.cellbio.23.090506.123521.
- Anastassiia Vertii; Alison Bright; Benedicte Delaval; Heidi Hehnly; Stephen Doxsey; New frontiers: discovering cilia‐independent functions of cilia proteins. EMBO reports 2015, 16, 1275-1287, 10.15252/embr.201540632.
- Kiet Hua; Russell J. Ferland; Primary cilia proteins: ciliary and extraciliary sites and functions. Cellular and Molecular Life Sciences 2018, 75, 1521-1540, 10.1007/s00018-017-2740-5.
- André Mourão; Søren T Christensen; Esben Lorentzen; The intraflagellar transport machinery in ciliary signaling. Current Opinion in Structural Biology 2016, 41, 98-108, 10.1016/j.sbi.2016.06.009.
- Maxence V Nachury; The molecular machines that traffic signaling receptors into and out of cilia. Current Opinion in Cell Biology 2018, 51, 124-131, 10.1016/j.ceb.2018.03.004.
- Barbara E. Tanos; Hui-Ju Yang; Rajesh Soni; Won-Jing Wang; Frank P. Macaluso; John M. Asara; Meng-Fu Bryan Tsou; Centriole distal appendages promote membrane docking, leading to cilia initiation. Genes & Development 2013, 27, 163-168, 10.1101/gad.207043.112.
- Marion Failler; Heon Yung Gee; Pauline Krug; Kwangsic Joo; Jan Halbritter; Lilya Belkacem; Emilie Filhol; Jonathan D. Porath; Daniela Anne Braun; Markus Schueler; et al. Mutations of CEP83 Cause Infantile Nephronophthisis and Intellectual Disability. The American Journal of Human Genetics 2014, 94, 905-914, 10.1016/j.ajhg.2014.05.002.
- Chien-Hui Lo; I-Hsuan Lin; T. Tony Yang; Yen-Chun Huang; Barbara Tanos; Po-Chun Chou; Chih-Wei Chang; Yeou-Guang Tsay; Jung-Chi Liao; Won-Jing Wang; et al. Phosphorylation of CEP83 by TTBK2 is necessary for cilia initiation. Journal of Cell Biology 2019, 218, 3489-3505, 10.1083/jcb.201811142.
- Jeffrey C. Nolz; Timothy S. Gomez; Peimin Zhu; Shuixing Li; Ricardo B. Medeiros; Yoji Shimizu; Janis K. Burkhardt; Bruce D. Freedman; Daniel D. Billadeau; The WAVE2 Complex Regulates Actin Cytoskeletal Reorganization and CRAC-Mediated Calcium Entry during T Cell Activation. Current Biology 2006, 16, 24-34, 10.1016/j.cub.2005.11.036.
- Audrey Le Floc’H; Yoshihiko Tanaka; Niels S. Bantilan; Guillaume Voisinne; Grégoire Altan-Bonnet; Yoshinori Fukui; Morgan Huse; Annular PIP3 accumulation controls actin architecture and modulates cytotoxicity at the immunological synapse. Journal of Experimental Medicine 2013, 210, 2721-2737, 10.1084/jem.20131324.
- Rania Ghossoub; Qicong Hu; Marion Failler; Marie-Christine Rouyez; Benjamin Spitzbarth; Serge Mostowy; Uwe Wolfrum; Sophie Saunier; Pascale Cossart; W. James Nelson; et al. Septins 2, 7, and 9 and MAP4 co-localize along the axoneme in the primary cilium and control ciliary length. Journal of Cell Science 2013, 126, 2583-2594, 10.1242/jcs.111377.
- Satyabrata Sinha; Marycharmain Belcastro; Poppy Datta; Seongjin Seo; Maxim Sokolov; Essential Role of the Chaperonin CCT in Rod Outer Segment Biogenesis. Investigative Opthalmology & Visual Science 2014, 55, 3775-3785, 10.1167/iovs.14-13889.
- Eugenio Bustos-Morán; Noelia Blas-Rus; Noa Martin-Cofreces; Francisco Sánchez-Madrid; Microtubule associated protein-4 (MAP4) controls nanovesicle dynamics and T cell activation. Journal of Cell Science 2017, 130, 1217-1223, 10.1242/jcs.199042.
- N. B. Martin-Cofreces; F. J. Chichon; E. Calvo; D. Torralba; E. Bustos-Moran; S. G. Dosil; A. Rojas-Gomez; E. Bonzon-Kulichenko; J. A. Lopez; J. Otón; et al. The chaperonin CCT controls T cell receptor–driven 3D configuration of centrioles. Science Advances 2020, 6, eabb7242, 10.1126/sciadv.abb7242.
- Anna Onnis; Cosima T. Baldari; Orchestration of Immunological Synapse Assembly by Vesicular Trafficking. Frontiers in Cell and Developmental Biology 2019, 7, 110, 10.3389/fcell.2019.00110.
- Maxence V. Nachury; Alexander V. Loktev; Qihong Zhang; Christopher J. Westlake; Johan Peränen; Andreas Merdes; Diane Slusarski; Richard H. Scheller; J. Fernando Bazan; Val Sheffield; et al. A Core Complex of BBS Proteins Cooperates with the GTPase Rab8 to Promote Ciliary Membrane Biogenesis. Cell 2007, 129, 1201-1213, 10.1016/j.cell.2007.03.053.
- Sebiha Cevik; Yuji Hori; Oktay I. Kaplan; Katarzyna Kida; Tiina Toivenon; Christian Foley-Fisher; David Cottell; Toshiaki Katada; Kenji Kontani; Oliver E. Blacque; et al. Joubert syndrome Arl13b functions at ciliary membranes and stabilizes protein transport in Caenorhabditis elegans. Journal of Cell Biology 2010, 188, 953-969, 10.1083/jcb.200908133.
- Oktay I. Kaplan; David B. Doroquez; Sebiha Cevik; Rachel V. Bowie; Lara Clarke; Anna A.W.M. Sanders; Katarzyna Kida; Joshua Z. Rappoport; Piali Sengupta; Oliver E. Blacque; et al. Endocytosis Genes Facilitate Protein and Membrane Transport in C. elegans Sensory Cilia. Current Biology 2012, 22, 451-460, 10.1016/j.cub.2012.01.060.
- Christopher J. Westlake; Lisa M. Baye; Maxence V. Nachury; Kevin J. Wright; Karen E. Ervin; Lilian Phu; Cecile Chalouni; John S. Beck; Donald S. Kirkpatrick; Diane C. Slusarski; et al. Primary cilia membrane assembly is initiated by Rab11 and transport protein particle II (TRAPPII) complex-dependent trafficking of Rabin8 to the centrosome. Proceedings of the National Academy of Sciences 2011, 108, 2759-2764, 10.1073/pnas.1018823108.
- Kevin J. Wright; Lisa M. Baye; Anique Olivier-Mason; Saikat Mukhopadhyay; Liyun Sang; Mandy Kwong; Weiru Wang; Pamela R. Pretorius; Val C. Sheffield; Piali Sengupta; et al. An ARL3–UNC119–RP2 GTPase cycle targets myristoylated NPHP3 to the primary cilium. Genes & Development 2011, 25, 2347-2360, 10.1101/gad.173443.111.
- Nele Schwarz; Alison Hardcastle; Michael E. Cheetham; Arl3 and RP2 mediated assembly and traffic of membrane associated cilia proteins. Vision Research 2012, 75, 2-4, 10.1016/j.visres.2012.07.016.
- Duarte C. Barral; Salil Garg; Cristina Casalou; Gerald F. M. Watts; José L. Sandoval; José S. Ramalho; Victor W. Hsu; Michael B. Brenner; Arl13b regulates endocytic recycling traffic. Proceedings of the National Academy of Sciences 2012, 109, 21354-21359, 10.1073/pnas.1218272110.
- Jing Wang; Dusanka Deretic; The Arf and Rab11 effector FIP3 acts synergistically with ASAP1 to direct Rabin8 in ciliary receptor targeting. Journal of Cell Science 2015, 128, 1375-1385, 10.1242/jcs.162925.
- Anna Onnis; F Finetti; Laura Patrussi; Marco Gottardo; Chiara Cassioli; Stefania Spano; C T Baldari; The small GTPase Rab29 is a common regulator of immune synapse assembly and ciliogenesis. Cell Death & Differentiation 2015, 22, 1687-1699, 10.1038/cdd.2015.17.
- Christina M. Szalinski; Anatália Labilloy; Jennifer R. Bruns; Ora A. Weisz; VAMP7 Modulates Ciliary Biogenesis in Kidney Cells. PLOS ONE 2014, 9, e86425, 10.1371/journal.pone.0086425.
- Jacob Morville Schrøder; Linda Schneider; Søren Tvorup Christensen; Lotte B. Pedersen; EB1 Is Required for Primary Cilia Assembly in Fibroblasts. Current Biology 2007, 17, 1134-1139, 10.1016/j.cub.2007.05.055.
- Jacob M. Schrøder; Jesper Larsen; Yulia Komarova; Anna Akhmanova; Rikke I. Thorsteinsson; Ilya Grigoriev; Robert Manguso; Søren T. Christensen; Stine F. Pedersen; Stefan Geimer; et al. EB1 and EB3 promote cilia biogenesis by several centrosome-related mechanisms. Development 2011, 138, e1608-e1608, 10.1242/dev.072231.
- Vinita Takiar; Kavita Mistry; Monica Carmosino; Nicole Schaeren-Wiemers; Michael J. Caplan; VIP17/MAL expression modulates epithelial cyst formation and ciliogenesis. American Journal of Physiology-Cell Physiology 2012, 303, C862-C871, 10.1152/ajpcell.00338.2011.
- Magdalena Cardenas-Rodriguez; Daniel Osborn; Florencia Irigoín; Martín Graña; Héctor Romero; Philip L. Beales; Jose L. Badano; Characterization of CCDC28B reveals its role in ciliogenesis and provides insight to understand its modifier effect on Bardet–Biedl syndrome. Quality of Life Research 2012, 132, 91-105, 10.1007/s00439-012-1228-5.
- Limin Hao; Jonathan M. Scholey; Intraflagellar transport at a glance. Journal of Cell Science 2009, 122, 889-892, 10.1242/jcs.023861.
- Olga M. Antón; Laura Andrés-Delgado; Natalia Reglero-Real; Alicia Batista; Miguel A. Alonso; MAL Protein Controls Protein Sorting at the Supramolecular Activation Cluster of Human T Lymphocytes. The Journal of Immunology 2011, 186, 6345-6356, 10.4049/jimmunol.1003771.
- Noa Martin-Cofreces; Francesc Baixauli; María J. López; Diana Gil; Alicia Monjas; Balbino Alarcón; Francisco Sánchez-Madrid; End-binding protein 1 controls signal propagation from the T cell receptor. The EMBO Journal 2012, 31, 4140-4152, 10.1038/emboj.2012.242.
- Helena Soares; Ricardo Henriques; Martin Sachse; Leandro Ventimiglia; Miguel A Alonso; Christophe Zimmer; Maria-Isabel Thoulouze; Andres Alcover; Regulated vesicle fusion generates signaling nanoterritories that control T-cell activation at the immunological synapse. Journal of Cell Biology 2013, 203, 2031OIA112-2031, 10.1083/jcb.2031oia112.
- Paola Larghi; David Williamson; Jean-Marie Carpier; Stephanie Dogniaux; Karine Chemin; Armelle Bohineust; Lydia Danglot; Katharina Gaus; Thierry Galli; Claire Hivroz; et al. VAMP7 controls T cell activation by regulating the recruitment and phosphorylation of vesicular Lat at TCR-activation sites. Nature Immunology 2013, 14, 723-731, 10.1038/ni.2609.
- Francesca Finetti; Laura Patrussi; Giulia Masi; Anna Onnis; Donatella Galgano; Orso Maria Lucherini; Gregory Pazour; Cosima T. Baldari; Immune synapse targeting of specific recycling receptors by the intraflagellar transport system. Journal of Cell Science 2014, 127, 1924-1937, 10.1242/jcs.139337.
- Francesca Finetti; Laura Patrussi; Donatella Galgano; Chiara Cassioli; Giuseppe Perinetti; Gregory Pazour; Cosima T. Baldari; The small GTPase Rab8 interacts with VAMP-3 to regulate the delivery of recycling TCRs to the immune synapse. Journal of Cell Science 2015, 128, 2541-2552, 10.1242/jcs.171652.
- Jérôme Bouchet; Iratxe del Río‐Iñiguez; Rémi Lasserre; Sonia Agüera‐Gonzalez; Céline Cuche; Anne Danckaert; Mary W McCaffrey; Vincenzo Di Bartolo; Andrés Alcover; Rac1‐Rab11‐ FIP 3 regulatory hub coordinates vesicle traffic with actin remodeling and T‐cell activation. The EMBO Journal 2016, 35, 1160-1174, 10.15252/embj.201593274.
- Jérôme Bouchet; Iratxe Del Río-Iñiguez; Elena Vázquez-Chávez; Rémi Lasserre; Sonia Agüera-González; Céline Cuche; Mary W. McCaffrey; Vincenzo Di Bartolo; Andrés Alcover; Rab11-FIP3 Regulation of Lck Endosomal Traffic Controls TCR Signal Transduction. The Journal of Immunology 2017, 198, 2967-2978, 10.4049/jimmunol.1600671.
- Louise A. Stephen; Yasmin ElMaghloob; Michael J. McIlwraith; Tamas Yelland; Patricia Castro Sanchez; Pedro Roda-Navarro; Shehab Ismail; The Ciliary Machinery Is Repurposed for T Cell Immune Synapse Trafficking of LCK. Developmental Cell 2018, 47, 122-132.e4, 10.1016/j.devcel.2018.08.012.
- Nagaja Capitani; Anna Onnis; Francesca Finetti; Chiara Cassioli; Alessandro Plebani; Jlenia Brunetti; Arianna Troilo; Sofia D’Elios; Manuela Baronio; Luisa Gazzurelli; et al. A CVID-associated variant in the ciliogenesis protein CCDC28B disrupts immune synapse assembly. Cell Death & Differentiation 2021, 29, 65-81, 10.1038/s41418-021-00837-5.
- Fiona Bangs; Kathryn V. Anderson; Primary Cilia and Mammalian Hedgehog Signaling. Cold Spring Harbor Perspectives in Biology 2016, 9, a028175, 10.1101/cshperspect.a028175.
- Emily K Ho; Tim Stearns; Hedgehog signaling and the primary cilium: implications for spatial and temporal constraints on signaling.. null 2021, 148, dev195552, .
- Maike de la Roche; Alex T. Ritter; Karen L. Angus; Colin Dinsmore; Charles H. Earnshaw; Jeremy F. Reiter; Gillian M. Griffiths; Hedgehog Signaling Controls T Cell Killing at the Immunological Synapse. Science 2013, 342, 1247-1250, 10.1126/science.1244689.