Polyploidy or whole-genome duplication (WGD) is widespread in nature, agriculture and aquaculture, normal physiology, regeneration, aging, and pathology. WGD results from the premature termination of the cell cycle or cell fusion. If WGD occurs in germ cells, the progeny organisms become completely polyploid, if in somatic cells, the somatic polyploidy arises in certain tissues of a given organism. Polyploidization leads to long-term consequences both in evolution (organismal) and ontogenesis (somatic). In evolution, WGD is one of the main sources for the growth of organismal complexity and evolutionary plasticity
- evolutionary conserved features
- complexity
- epigenetic changes
- biological plasticity
- adaptation
- stress resistance
- regeneration
- carcinogenesis
- aging
- developmental programming
- cardiovascular disease
1. Introduction
2. Evolution
2.1. General Picture
2.2. Cellular and Molecular Aspects
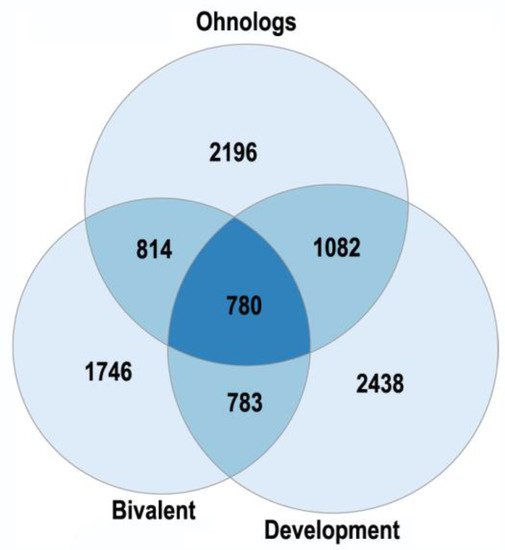
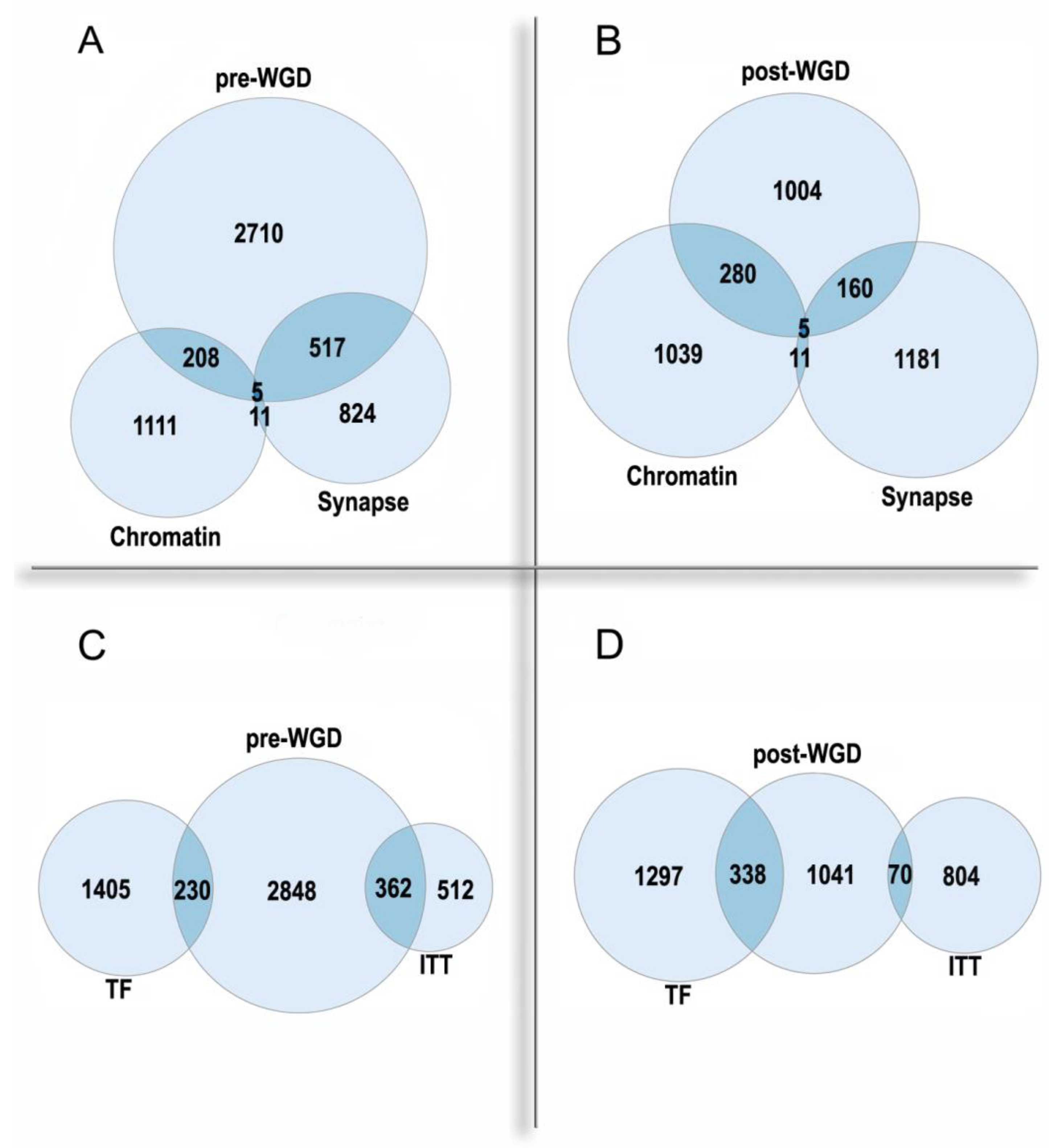
2.3. Polyploidy in Agriculture and Aquaculture Biotechnology
This entry is adapted from the peer-reviewed paper 10.3390/ijms23073542
References
- Gjelsvik, K.J.; Besen-McNally, R.; Losick, V.P. Solving the Polyploid Mystery in Health and Disease. Trends Genet. 2019, 35, 6–14.
- Donne, R.; Saroul-Aïnama, M.; Cordier, P.; Celton-Morizur, S.; Desdouets, C. Polyploidy in Liver Development, Homeostasis and Disease. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 391–405.
- Fox, D.T.; Soltis, D.E.; Soltis, P.S.; Ashman, T.-L.; Van de Peer, Y. Polyploidy: A Biological Force From Cells to Ecosystems. Trends Cell Biol. 2020, 30, 688–694.
- Dörnen, J.; Sieler, M.; Weiler, J.; Keil, S.; Dittmar, T. Cell Fusion-Mediated Tissue Regeneration as an Inducer of Polyploidy and Aneuploidy. Int. J. Mol. Sci. 2020, 21, 1811.
- Van de Peer, Y.; Mizrachi, E.; Marchal, K. The Evolutionary Significance of Polyploidy. Nat. Rev. Genet. 2017, 18, 411–424.
- Van de Peer, Y.; Ashman, T.-L.; Soltis, P.S.; Soltis, D.E. Polyploidy: An Evolutionary and Ecological Force in Stressful Times. Plant Cell 2021, 33, 11–26.
- Anatskaya, O.V.; Vinogradov, A.E. Genome Multiplication as Adaptation to Tissue Survival: Evidence from Gene Expression in Mammalian Heart and Liver. Genomics 2007, 89, 70–80.
- Anatskaya, O.V.; Vinogradov, A.E. Somatic Polyploidy Promotes Cell Function under Stress and Energy Depletion: Evidence from Tissue-Specific Mammal Transcriptome. Funct. Integr. Genom. 2010, 10, 433–446.
- Anatskaya, O.V.; Sidorenko, N.V.; Beyer, T.V.; Vinogradov, A.E. Neonatal Cardiomyocyte Ploidy Reveals Critical Windows of Heart Development. Int. J. Cardiol. 2010, 141, 81–91.
- Anatskaya, O.V.; Erenpreisa, J.A.; Salmina, K.A.; Vazquez-Martin, A.; Huna, A.; Nikolsky, N.N.; Vinogradov, A.E. Polyploidy Activates Biological Pathways Related to Morphogenesis in Mammalian Tissues. MOJ Immunol. 2018, 6, 90–93.
- Vazquez-Martin, A.; Anatskaya, O.V.; Giuliani, A.; Erenpreisa, J.; Huang, S.; Salmina, K.; Inashkina, I.; Huna, A.; Nikolsky, N.N.; Vinogradov, A.E. Somatic Polyploidy Is Associated with the Upregulation of C-MYC Interacting Genes and EMT-like Signature. Oncotarget 2016, 7, 75235–75260.
- Vinogradov, A.E.; Anatskaya, O.V. Evolutionary Framework of the Human Interactome: Unicellular and Multicellular Giant Clusters. Biosystems 2019, 181, 82–87.
- Ohno, S. Evolution by Gene Duplication; Springer: Berlin/Heidelberg, Germany, 1970; ISBN 978-3-642-86661-6.
- Singh, P.P.; Isambert, H. OHNOLOGS v2: A Comprehensive Resource for the Genes Retained from Whole Genome Duplication in Vertebrates. Nucleic Acids Res. 2020, 48, D724–D730.
- Inoue, J.; Sato, Y.; Sinclair, R.; Tsukamoto, K.; Nishida, M. Rapid Genome Reshaping by Multiple-Gene Loss after Whole-Genome Duplication in Teleost Fish Suggested by Mathematical Modeling. Proc. Natl. Acad. Sci. USA 2015, 112, 14918–14923.
- Robertson, F.M.; Gundappa, M.K.; Grammes, F.; Hvidsten, T.R.; Redmond, A.K.; Lien, S.; Martin, S.A.M.; Holland, P.W.H.; Sandve, S.R.; Macqueen, D.J. Lineage-Specific Rediploidization Is a Mechanism to Explain Time-Lags between Genome Duplication and Evolutionary Diversification. Genome Biol. 2017, 18, 111.
- Soppa, J. Polyploidy and Community Structure. Nat. Microbiol. 2017, 2, 16261.
- Hu, G.; Wendel, J.F. Cis-Trans Controls and Regulatory Novelty Accompanying Allopolyploidization. New Phytol. 2019, 221, 1691–1700.
- Evans, B.J.; Carter, T.F.; Greenbaum, E.; Gvoždík, V.; Kelley, D.B.; McLaughlin, P.J.; Pauwels, O.S.G.; Portik, D.M.; Stanley, E.L.; Tinsley, R.C.; et al. Genetics, Morphology, Advertisement Calls, and Historical Records Distinguish Six New Polyploid Species of African Clawed Frog (Xenopus, Pipidae) from West and Central Africa. PLoS ONE 2015, 10, e0142823.
- Du, K.; Stöck, M.; Kneitz, S.; Klopp, C.; Woltering, J.M.; Adolfi, M.C.; Feron, R.; Prokopov, D.; Makunin, A.; Kichigin, I.; et al. The Sterlet Sturgeon Genome Sequence and the Mechanisms of Segmental Rediploidization. Nat. Ecol. Evol. 2020, 4, 841–852.
- Moritz, C.; Bi, K. Spontaneous Speciation by Ploidy Elevation: Laboratory Synthesis of a New Clonal Vertebrate. Proc. Natl. Acad. Sci. USA 2011, 108, 9733–9734.
- Otto, S.P. The Evolutionary Consequences of Polyploidy. Cell 2007, 131, 452–462.
- Evans, B.J.; Upham, N.S.; Golding, G.B.; Ojeda, R.A.; Ojeda, A.A. Evolution of the Largest Mammalian Genome. Genome Biol. Evol. 2017, 9, 1711–1724.
- Barker, M.S.; Arrigo, N.; Baniaga, A.E.; Li, Z.; Levin, D.A. On the Relative Abundance of Autopolyploids and Allopolyploids. New Phytol. 2016, 210, 391–398.
- Venkatachalam, A.B.; Parmar, M.B.; Wright, J.M. Evolution of the Duplicated Intracellular Lipid-Binding Protein Genes of Teleost Fishes. Mol. Genet. Genom. MGG 2017, 292, 699–727.
- Cheng, F.; Wu, J.; Cai, X.; Liang, J.; Freeling, M.; Wang, X. Gene Retention, Fractionation and Subgenome Differences in Polyploid Plants. Nat. Plants 2018, 4, 258–268.
- Vinogradov, A.E. Global versus Local Centrality in Evolution of Yeast Protein Network. J. Mol. Evol. 2009, 68, 192–196.
- Vinogradov, A.E.; Anatskaya, O.V. Loss of Protein Interactions and Regulatory Divergence in Yeast Whole-Genome Duplicates. Genomics 2009, 93, 534–542.
- Corrochano, L.M.; Kuo, A.; Marcet-Houben, M.; Polaino, S.; Salamov, A.; Villalobos-Escobedo, J.M.; Grimwood, J.; Álvarez, M.I.; Avalos, J.; Bauer, D.; et al. Expansion of Signal Transduction Pathways in Fungi by Extensive Genome Duplication. Curr. Biol. 2016, 26, 1577–1584.
- Court, F.; Arnaud, P. An Annotated List of Bivalent Chromatin Regions in Human ES Cells: A New Tool for Cancer Epigenetic Research. Oncotarget 2017, 8, 4110–4124.
- Jeon, A.-J.; Tucker-Kellogg, G. Bivalent Genes That Undergo Transcriptional Switching Identify Networks of Key Regulators of Embryonic Stem Cell Differentiation. BMC 2020, 21, 614.
- Vinogradov, A.E. “Genome Design” Model and Multicellular Complexity: Golden Middle. Nucleic Acids Res. 2006, 34, 5906–5914.
- Vinogradov, A.E.; Anatskaya, O.V. Growth of Biological Complexity from Prokaryotes to Hominids Reflected in the Human Genome. Int. J. Mol. Sci. 2021, 22, 11640.
- Hu, H.; Miao, Y.-R.; Jia, L.-H.; Yu, Q.-Y.; Zhang, Q.; Guo, A.-Y. AnimalTFDB 3.0: A Comprehensive Resource for Annotation and Prediction of Animal Transcription Factors. Nucleic Acids Res. 2019, 47, D33–D38.
- Mattenberger, F.; Sabater-Muñoz, B.; Toft, C.; Fares, M.A. The Phenotypic Plasticity of Duplicated Genes in Saccharomyces Cerevisiae and the Origin of Adaptations. G3: Genes Genomes Genet. 2017, 7, 63–75.
- Zhang, K.; Wang, X.; Cheng, F. Plant Polyploidy: Origin, Evolution, and Its Influence on Crop Domestication. Hortic. Plant J. 2019, 5, 231–239.
- Ruiz, M.; Quiñones, A.; Martínez-Cuenca, M.R.; Aleza, P.; Morillon, R.; Navarro, L.; Primo-Millo, E.; Martínez-Alcántara, B. Tetraploidy Enhances the Ability to Exclude Chloride from Leaves in Carrizo Citrange Seedlings. J. Plant Physiol. 2016, 205, 1–10.
- Bhatta, M.; Morgounov, A.; Belamkar, V.; Wegulo, S.N.; Dababat, A.A.; Erginbas-Orakci, G.; Bouhssini, M.E.; Gautam, P.; Poland, J.; Akci, N.; et al. Genome-Wide Association Study for Multiple Biotic Stress Resistance in Synthetic Hexaploid Wheat. Int. J. Mol. Sci. 2019, 20, 3667.
- Yao, Y.; Carretero-Paulet, L.; Van de Peer, Y. Using Digital Organisms to Study the Evolutionary Consequences of Whole Genome Duplication and Polyploidy. PLoS ONE 2019, 14, e0220257.
- Salman-Minkov, A.; Sabath, N.; Mayrose, I. Whole-Genome Duplication as a Key Factor in Crop Domestication. Nat. Plants 2016, 2, 16115.
- Glombik, M.; Bačovský, V.; Hobza, R.; Kopecký, D. Competition of Parental Genomes in Plant Hybrids. Front. Plant Sci. 2020, 11, 200.
- Guo, H.; Mendrikahy, J.N.; Xie, L.; Deng, J.; Lu, Z.; Wu, J.; Li, X.; Shahid, M.Q.; Liu, X. Transcriptome Analysis of Neo-Tetraploid Rice Reveals Specific Differential Gene Expressions Associated with Fertility and Heterosis. Sci. Rep. 2017, 7, 40139.
- Julião, S.A.; do Ribeiro, C.V.; Lopes, J.M.L.; de Matos, E.M.; Reis, A.C.; Peixoto, P.H.P.; Machado, M.A.; Azevedo, A.L.S.; Grazul, R.M.; de Campos, J.M.S.; et al. Induction of Synthetic Polyploids and Assessment of Genomic Stability in Lippia Alba. Front. Plant Sci. 2020, 11, 292.
- Zhou, L.; Gui, J. Natural and Artificial Polyploids in Aquaculture. Aquac. Fish. 2017, 2, 103–111.
- Vinogradov, A.E.; Borkin, L.J.; Günther, R.; Rosanov, J.M. Two Germ Cell Lineages with Genomes of Different Species in One and the Same Animal. Hereditas 1991, 114, 245–251.
- Vinogradov, A.E.; Borkin, L.J.; Günther, R.; Rosanov, J.M. Genome Elimination in Diploid and Triploid Rana Esculenta Males: Cytological Evidence from DNA Flow Cytometry. Genome 1990, 33, 619–627.
