A significant proportion of patients with COVID-19 pneumonia could develop acute respiratory distress syndrome (ARDS), thus requiring mechanical ventilation and resulting in a high rate of intensive care unit (ICU) admission. Several complications can arise during ICU stay, from both COVID-19 infection and respiratory supporting system, including barotraumas (pneumothorax and pneumomediastinum), superimposed pneumonia, coagulation disorders (pulmonary embolism, venous thromboembolism, hemorrhages, and acute ischemic stroke), abdominal involvement (acute mesenteric ischemia, pancreatitis, and acute kidney injury) and sarcopenia. Imaging plays a pivotal role in the detection and monitoring of ICU complications and is expanding even to prognosis prediction.
1. Pneumothorax
Pneumothorax represents the most common form of barotrauma
[1] and develops whenever alveolar pressure increases, resulting in the rupture of alveoli, air dissection along the pulmonary interstitium and eventually air spreading into the pleural space (Macklin effect)
[2].
The incidence of pneumothorax in intensive care unit (ICU) patients with COVID-19 is higher than what is reported for other ICU patients, ranging from 4% to 15%, regardless of the ventilatory strategies
[3][4][5]. Moreover, its frequency further increased in the case of prolonged mechanical ventilation or refractory respiratory insufficiency requiring extracorporeal membrane oxygenation (ECMO)
[6][7]. The occurrence of pneumothorax in ICU patients with COVID-19 has been associated with increased overall mortality
[8], therefore, its prompt and correct detection is fundamental.
Pneumothorax is usually secondary to mechanical ventilation with high positive end-expiratory pressure (PEEP) or the cannulation of subclavian or neck vessels
[9]. However, several
[10] have interestingly pointed out that about 33–38% of air leaks occurred in COVID-19 patients who never received ventilatory support, raising the question of whether these patients are more prone to develop air leaks than patients with acute respiratory distress syndrome (ARDS) for other causes.
With this regard, it has been speculated that the air-filled cysts developing in the diseased areas of the lungs as a late consequence of ARDS, especially those peripherally located, may progress to pneumatocele and then spontaneously rupture, thus explaining the significant number of air leaks occurring in patients who had never received ventilatory support
[4] . Therefore, baseline CT imaging may be predictive for patients who may develop pneumothorax
[10].
Chest X-ray is an easy-to-use imaging technique to evaluate patients on ventilators but, despite its high specificity (99%), has a low sensibility for diagnosing pneumothorax (38%)
[11].
Pneumothorax classically appears as a thin opaque line representing the visceral pleura outlined by lucent air on both sides, corresponding to the air in the pleural space on the chest wall side and in the lung parenchyma on the hilar side, respectively; other signs include the absence of distal lung markings and hypertranslucency of the pleural space
[12]. On the standard upright view, air typically starts to collect in the apical region and laterally more than medially. However, in most ICU patients with suspected barotrauma, only supine X-rays are possible, thus pneumothorax becomes more difficult to detect
[13]. In this position, the air is usually most visible at the anteromedial or subpulmonary location, outlines the structures of the mediastinum and may enlarge the costophrenic angle (the deep sulcus sign)
[11].
2. Pneumomediastinum, Pneumopericardium and Subcutaneous Emphysema
Pneumomediastinum has been identified as the second most common barotrauma-related event in patients with mechanical ventilation for COVID-19 ARDS
[2]. The mechanism of development of pneumomediastinum is the same as pneumothorax and is linked to the Macklin phenomenon, so much so that the association between these two entities has been widely reported
[14]. It can be either spontaneous or secondary to mechanical ventilation in COVID-19 patients and occurs when air leaks from ruptured alveoli into the mediastinum
[12]. Rarely, the released air can track to the subcutaneous tissue, causing subcutaneous emphysema (
Figure 1D), and/or to the pericardial tissue causing pneumopericardium
[2][15][16].
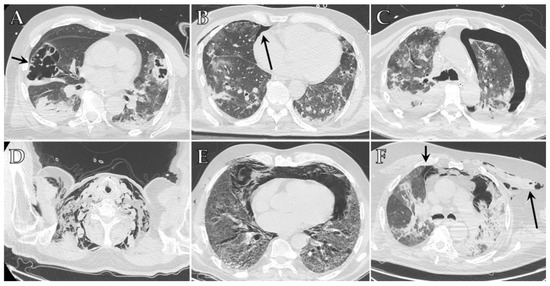
Figure 1. Axial HRCT images of different patients with COVID-19 ARDS admitted to ICU showing imaging features indicative of barotrauma secondary to mechanical ventilation: (A) large pneumatocele (black arrow); (B) small anterior pneumothorax (black arrow); (C) large left pneumothorax; (D) extensive bilateral subcutaneous emphysema of the neck; (E) pneumomediastinum; (F) pneumomediastinum associated with small anterior pneumothorax (small black arrow) and subcutaneous emphysema of the left upper chest (long black arrow).
The occurrence of pneumomediastinum in patients with COVID-19 ARDS is higher compared to patients who had ARDS due to other causes, despite protective mechanical ventilation strategies
[17], and is associated with a poorer outcome and an increased mortality rate
[18].
Pneumomediastinum can be identified on chest X-ray by radiolucent air surrounding the normal anatomic structures within the mediastinum, well demarcating the great vessel and the cardiac contour, especially along its left border. When air dissects inferiorly into the infracardiac region, it separates the cardiac and diaphragmatic densities, and the diaphragm can be seen in its entire extent
[19]. When multiple vertically oriented lucencies are visualized extending superiorly into the neck, subcutaneous emphysema must be suspected
[20]. Isolated pneumopericardium rarely occurs and manifests as a single band of gas that outlines the pericardium, especially along the left ventricle and right atrium contour
[11].
On chest CT, pneumomediastinum is visualized as gas outlining the structures within the mediastinum (
Figure 1E,F). Air collection in the extrapleural space may resemble pneumothorax, but the presence of thin connective tissue webs outside of the pleural line is typical of pneumomediastinum and can help achieve a correct differential diagnosis
[11]. Chest CT is performed to confirm the diagnosis in equivocal cases on chest X-ray, to assess the extent of the pneumomediastinum, and to evaluate the presence of pneumopericardium, which may have a less favorable prognosis and might require treatment
[21].
3. Ventilator-Associated Pneumonia (VAP) and Invasive Pulmonary Aspergillosis (IPA)
VAP is the most common ICU-acquired infection and is defined as an infection of pulmonary parenchyma developed in patients receiving mechanical ventilation for at least 48 h
[22]. Previous reports indicated that COVID-19 patients have an increased risk of VAP compared to other ARDS, with a variable incidence of 29–79% (vs. 13%), and present even higher mortality rates
[23][24]. The increased risk of VAP in ICU patients with COVID-19 is suspected to be due to multiple factors, including prolonged duration of mechanical ventilation, the extensive use of prone positioning, a higher risk for pulmonary infarction, disease, and therapy-associated immune impairment, possibly further amplified by ICU overcrowding during the pandemic outbreak
[23].
The most common causative pathogens of VAP are bacteria, which can colonize in the endotracheal/tracheostomy tube of ICU patients. Several have demonstrated that the distribution of infecting organisms is similar between patients with and without COVID-19, with Enterobacteria (mainly
Escherichia coli and
Klebsiella pneumoniae) representing the most frequent pathogens (in about 40% of cases), followed by other Gram-negative bacilli (such as
Pseudomonas aeruginosa) and Gram-positive cocci (such as
Staphylococcus aureus and
Streptococcus pneumoniae), both accounting for about another 25% of cases each
[25][26][27].
Diagnosis of VAP is challenging in COVID-19 patients, due to the overlap of VAP imaging features with those of worsening COVID-19 pneumonia, and laboratory results provide essential support to both clinicians and radiologists
[28]. Generally, a sudden increase in X-ray opacities should raise suspicion for VAP, especially if paired with rapid clinical deterioration of the patient
[29].
Chest CT is the imaging modality of choice to diagnose IA, as it can detect pulmonary nodules with surrounding ground-glass infiltrates (halo sign), which reflect angio-invasion and hemorrhage in the area surrounding the fungal infection. Sometimes these nodules may cavitate and a fungal ball can be seen within the cavity, with an air-crescent sign
[30][31].
Although frequently secondary to bacterial, fungal, or mycobacterial co-infections
[32][33][34], lung cavitation has also been reported as an atypical imaging feature of late stages of COVID-19 pneumonia
[35]. In particular, COVID-19 cavities may be related to barotraumas, pulmonary embolism and infarction with a subsequent necrotic lesion of the lung tissue, or simply to necrotic evolution of the denser consolidations, as well as to all these factors working together
[36].
Mechanically ventilated patients are also at increased risk of aspiration pneumonia (AP).
Worsening of X-ray opacities or the appearance of new ones, especially in the retrocardiac and infrahilar regions, should raise the suspicion of AP. Confirmation chest CT may demonstrate the presence of tree-in-bud, centrilobular nodules or consolidations in the dependent parts of the lungs, usually most pronounced in the lower lobes in supine patients or in the upper lobes and right middle lobe in prone patients
[11].
4. Vascular Complications
The high prevalence of the thrombotic events in patients with COVID-19 may be induced by cross-talking between immune and coagulation systems since the high levels of inflammatory cytokines (such as IL-6 and TNF) induce activation of the endothelial cells and tissue factor, which triggers the coagulation cascade. Accordingly, D-dimer was reported as the most prominent factor in determining COVID-19 severity and subsequent complications
[37].
COVID-19 patients in ICU showed a significant increase in the cumulative incidence of symptomatic venous thromboembolism compared with those not requiring ICU care
[38], ranging between 31% and 69%
[39][40]. Similarly, the percentage of COVID-19 ICU patients that were diagnosed with pulmonary embolism (PE) was higher compared to non-COVID-19 patients, despite having a similar severity to ARDS (23–47% vs. 7.5%)
[41][40][42][43][44]. Moreover, thromboembolic complications have been described even in patients treated with anticoagulation therapy from admission, highlighting the intrinsic thrombogenicity of COVID-19
[40]. Due to prolonged immobility, mechanical ventilation and the use of sedatives and neuromuscular blockers, ICU patients experience a significant flow stasis, which could explain their increased risk for thromboembolic complications. Moreover, the frequent insertion of central venous catheters may cause vessel injury, which can further precipitate thrombotic risk in these vulnerable patients
[45]. Finally, ECMO may significantly perturb the normal balance of hemostasis with biomaterial-mediated activation of coagulation, complement and inflammatory cascades, as well as increased platelet activation
[46][47].
5. Gastrointestinal Complications
Intestinal involvement related to COVID-19 results from either a direct viral infection, virus-induced inflammatory response, or bowel wall ischemia due to disease hypercoagulability
[48][49]. Mesenteric ischemia, ileus, colitis and terminal ileitis are the most common gastrointestinal complications.
The prevalence of gastrointestinal symptoms and complications in ICU patients with severe COVID-19 is higher compared with those with mild disease (16.6% vs. 11.7%, respectively)
[50][51][52][53] and with ICU patients without COVID-19
[54].
CT represents the gold standard for definitive diagnosis of acute mesenteric ischemia, since the US may be limited in obese patients and in the case of pneumoperitoneum or an excessive amount of bowel gas. Acute mesenteric ischemia appears as a filling defect within the lumen of the abdominal aorta, the celiac axis, the superior mesenteric artery and/or the inferior mesenteric artery, sometimes with poor contrast opacification of the mesenteric vascular arcade, which is indicative of hypoperfusion and is better seen on maximum intensity projection (MIP). Accordingly, the segment of the injured small bowel reveals absent or decreased contrast enhancement and wall thickening, with target appearance due to mural edema. In ICU patients, most cases of acute mesenteric ischemia involve the large bowel alone (56%) and less frequently, both the large and small bowel (24%)
[55]. The early phase of bowel ischemia may only show contracted gasless bowel that, when progressed, may transform into dilated intestinal loops with a paper-thin wall and air-fluid levels. In the late phase, transmural infarction may lead to intestinal wall pneumatosis, mesenteric venous gas, pneumoperitoneum due to perforation, and free fluid in the abdominal cavity
[49][51][56].
Imaging findings suggestive of viral colitis or terminal ileitis include circumferential bowel wall thickening with edema, mucosal hyperenhancement, mesenteric hypervascularity, fluid-filled mildly distended intestinal lumen and pericolic fat stranding, and in absence of filling defects is suggestive of thrombi in the abdominal arteries
[50].
Pancreatic damage has been observed in 1–2% of mild cases and 17% of severe COVID-19 cases, including patients in ICU
[57]. It has been speculated that pancreatic injury could be a consequence of both viral cytotoxicity on pancreatic islet cells and the severe immune response triggered by the infection
[58]. Despite this, in most of these reports, the diagnosis was made only based on the elevation of pancreatic enzymes levels, which can be secondary to a multitude of causes, and radiological findings were missing. Cases of acute pancreatitis related to COVID-19 without additional pathology in the etiology have been described
[59][60], including necrotizing pancreatitis
[61].
6. Renal Complications
Patients with COVID-19 could experience high rates of acute kidney injury (AKI), especially those in ICU, with rates of approximately 20–40%
[62][63][64].
Since ACE-2 receptors are highly expressed in kidney tubules, renal injury is most likely caused by a direct virus-induced cytopathic effect, with acute tubular necrosis, interstitial inflammation and protein leakage in the Bowman’s capsule
[65][66]; however, a systemic cytokine storm developing in severely ill patients may also play a role
[67]. Moreover, there have been several reports describing renal infarcts due to the severe hypercoagulability state which can occur in COVID-19 patients
[68].
AKI during COVID-19 infection is considered a negative prognostic factor for survival and patients may require renal replacement therapy
[69].
Abdominal CT with acquisition during the arterial and venous phases can easily detect AKI, showing the enlarged kidney with a loss of corticomedullary differentiation. Renal infarctions appear as solitary or multiple, triangular-shaped areas of parenchyma with decreased perfusion and/or enhancement, with the apex pointing towards the medulla and base parallel to the subcapsular region
[49][68][70]. When a filling defect in the aorta or the renal arteries is not visible, microthrombi may be suspected, as they have been documented on histopathological samples
[71].
7. Neurological Complications
Acute cerebrovascular events, such as acute ischemic stroke and intracranial hemorrhage (intraparenchymal and subarachnoid) are the most common neurological complications of COVID-19, but meningoencephalitis, encephalopathy and encephalomyelitis have also been reported
[72].
COVID-19 neurological complications appear to involve both ischemic and hemorrhagic coagulation disturbances secondary to direct viral invasion through olfactory pathways or the bloodstream, with consequent endothelial damage
[73]. Moreover, the exacerbated inflammatory response with the hypersecretion of cytokines, together with prolonged hypoxemia and a form of small vessel vasculitis, further increases the risk of acute cerebrovascular complications
[74][75]. This could explain why cerebrovascular complications seem to affect patients with COVID-19 that are younger and have no history of vascular abnormality compared to patients with ischemic stroke/intracranial hemorrhages due to other causes
[72].
Neurologic symptoms have been reported to be more common in patients admitted to ICU (84%), and are associated with a worse prognosis compared to patients with normal neurological examination
[76]. Acute neurologic findings were recorded in 14% of patients on admission to the ICU
[77], and stroke was demonstrated to be more frequent in this scenario compared to hospitalized patients (5–6% vs. 1–3%)
[77][78]. In addition, despite the mortality benefit of ECMO demonstrated in patients with ARDS, the systemic anticoagulation required to reduce circuit clotting further increases the risk of neurologic complications, especially extensive intracerebral hemorrhages
[79][80][81].
Imaging features of acute ischemic stroke in COVID-19 patients are similar to those described in non-COVID-19 patients, including abnormal hypoattenuation of the brain parenchyma, a loss of gray-white differentiation, and sulcal effacement on unenhanced computed tomography (CT). After contrast media administration, an extensive vascular occlusive disease may be revealed, typically involving the large vessel
[82].
Intracerebral hemorrhages may occur spontaneously in critically ill patients, particularly in the context of circulation instability, or as a hemorrhagic transformation of acute ischemic stroke. Hemorrhages in COVID-19 patients resemble those secondary to anticoagulant therapy, appearing as a markedly hyperdense area with surrounding edema at unenhanced CT. In severely ill ICU patients, hemorrhages may be massive, with extensive hemispheric involvement and/or with multiple hematomas occurring in both supra and infra-tentorial locations, sometimes with intraventricular extension
[78][83] .
Finally, recent studies have analyzed brain MRIs of critically ill patients with COVID-19 after their discharge and described diffuse leuko-encephalopathy and microhemorrhages as late complications of the prolonged hypoxemia and/or the hyperinflammatory state in about 25% of cases
[84][85]. It was suggest that COVID-19 may produce protracted neurological sequelae, but further are needed to confirm these results.
8. Sarcopenia
It has been reported that ICU patients with COVID-19 show significant reductions in skeletal muscle mass and strength during their hospitalization
[86][87], with increased morbidity and mortality and persistence in about one-third of cases, even post-discharge
[88].
The acute inflammatory state and the procoagulant state described in the severe form of COVID-19 may play a role in the onset of sarcopenia, causing an increased glucocorticoid and catecholamine production with consequent hypercatabolism and anabolic resistance
[89][90]. This process perturbs muscle homeostasis and decreases muscle quality and quantity, especially at the level of respiratory muscles, impairing the ability to produce appropriate tidal volumes and to perform high force expulsive airway clearance maneuvers
[91].
CT is considered the gold standard for investigating quantitative and qualitative changes in muscle and fat, especially in the trunk area. In fact, besides the mere quantification of the muscle mass, CT can evaluate the quality of muscle based on identifying the fat portion within the muscle through the evaluation of specific attenuation
[92].
Giraudo et al.
[93] have recently demonstrated that a value above 34 HU in the right paravertebral muscle at the level of T12 is a highly sensitive CT prognostic factor for ICU admission in COVID-19 patients. Similarly, Schiaffino et al.
[87] reported that paravertebral skeletal muscle mass at T12 and, especially, T5, can predict ICU admission and death. Finally, Damanti et al.
[94] have demonstrated that muscle quality perturbations were predictive of the length of hospitalization and in-hospital mortality, as well as the need for reintubation after the discontinuation of mechanical ventilation.
This entry is adapted from the peer-reviewed paper 10.3390/diagnostics12040846