Clinical trials for Alzheimer’s disease (AD) face multiple challenges, such as the high screen failure rate and the even allocation of heterogeneous participants. Artificial intelligence (AI), which has become a potent tool of modern science with the expansion in the volume, variety, and velocity of biological data, offers promising potential to address these issues in AD clinical trials. The current status of AD clinical trials and the topic of machine learning were introduced. Then, a comprehensive review is focused on the potential applications of AI in the steps of AD clinical trials, including the prediction of protein and MRI AD biomarkers in the prescreening process during eligibility assessment and the likelihood stratification of AD subjects into rapid and slow progressors in randomization. Finally, this review provides challenges, developments, and the future outlook on the integration of AI into AD clinical trials.
- Alzheimer’s disease
- artificial intelligence
- clinical trials
- eligibility assessment
- randomization
1. Introduction
2. Eligibility Assessment
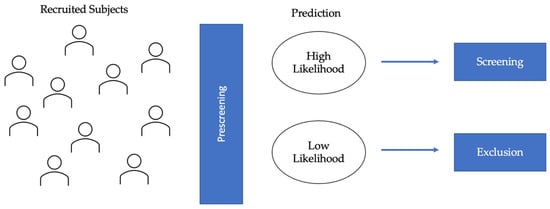
2.1. Protein Biomarkers for AD
2.2. MRI Biomarkers
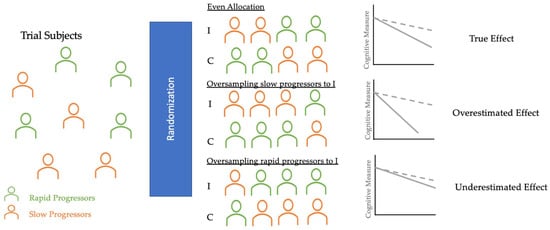
3. Randomization
4. Challenges and Future Directions
5. Conclusions
This entry is adapted from the peer-reviewed paper 10.3390/life12020275
References
- Ritchie, C.W.; Molinuevo, J.L.; Truyen, L.; Satlin, A.; Van der Geyten, S.; Lovestone, S.; European Prevention of Alzheimer’s Dementia Consortium. Development of interventions for the secondary prevention of Alzheimer’s dementia: The European Prevention of Alzheimer’s Dementia (EPAD) project. Lancet Psychiatry 2016, 3, 179–186.
- Leal, S.L.; Lockhart, S.N.; Maass, A.; Bell, R.K.; Jagust, W.J. Subthreshold Amyloid Predicts Tau Deposition in Aging. J. Neurosci. 2018, 38, 4482–4489.
- Karran, E.; Mercken, M.; De Strooper, B. The amyloid cascade hypothesis for Alzheimer’s disease: An appraisal for the development of therapeutics. Nat. Rev. Drug Discov. 2011, 10, 698–712.
- Jack, C.R., Jr.; Knopman, D.S.; Jagust, W.J.; Petersen, R.C.; Weiner, M.W.; Aisen, P.S.; Shaw, L.M.; Vemuri, P.; Wiste, H.J.; Weigand, S.D.; et al. Tracking pathophysiological processes in Alzheimer’s disease: An updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013, 12, 207–216.
- Hardy, J.A.; Higgins, G.A. Alzheimer’s disease: The amyloid cascade hypothesis. Science 1992, 256, 184–185.
- Giannakopoulos, P.; Herrmann, F.R.; Bussiere, T.; Bouras, C.; Kovari, E.; Perl, D.P.; Morrison, J.H.; Gold, G.; Hof, P.R. Tangle and neuron numbers, but not amyloid load, predict cognitive status in Alzheimer’s disease. Neurology 2003, 60, 1495–1500.
- Mangialasche, F.; Solomon, A.; Winblad, B.; Mecocci, P.; Kivipelto, M. Alzheimer’s disease: Clinical trials and drug development. Lancet Neurol. 2010, 9, 702–716.
- Cummings, J.; Aisen, P.; Lemere, C.; Atri, A.; Sabbagh, M.; Salloway, S. Aducanumab produced a clinically meaningful benefit in association with amyloid lowering. Alzheimer’s Res. 2021, 13, 98.
- DeCarli, C. Mild cognitive impairment: Prevalence, prognosis, aetiology, and treatment. Lancet Neurol. 2003, 2, 15–21.
- Nettiksimmons, J.; Harvey, D.; Brewer, J.; Carmichael, O.; DeCarli, C.; Jack, C.R., Jr.; Petersen, R.; Shaw, L.M.; Trojanowski, J.Q.; Weiner, M.W.; et al. Subtypes based on cerebrospinal fluid and magnetic resonance imaging markers in normal elderly predict cognitive decline. Neurobiol. Aging 2010, 31, 1419–1428.
- Bali, J.; Bali, O. Artificial intelligence in ophthalmology and healthcare: An updated review of the techniques in use. Indian J. Ophthalmol. 2021, 69, 8–13.
- McKhann, G.; Drachman, D.; Folstein, M.; Katzman, R.; Price, D.; Stadlan, E.M. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984, 34, 939–944.
- McKhann, G.M.; Knopman, D.S.; Chertkow, H.; Hyman, B.T.; Jack, C.R., Jr.; Kawas, C.H.; Klunk, W.E.; Koroshetz, W.J.; Manly, J.J.; Mayeux, R.; et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 2011, 7, 263–269.
- Landau, S.M.; Horng, A.; Fero, A.; Jagust, W.J.; Alzheimer’s Disease Neuroimaging Initiative. Amyloid negativity in patients with clinically diagnosed Alzheimer disease and MCI. Neurology 2016, 86, 1377–1385.
- Sevigny, J.; Suhy, J.; Chiao, P.; Chen, T.; Klein, G.; Purcell, D.; Oh, J.; Verma, A.; Sampat, M.; Barakos, J. Amyloid PET Screening for Enrichment of Early-Stage Alzheimer Disease Clinical Trials: Experience in a Phase 1b Clinical Trial. Alzheimer Dis. Assoc. Disord. 2016, 30, 1–7.
- Chetelat, G.; La Joie, R.; Villain, N.; Perrotin, A.; de La Sayette, V.; Eustache, F.; Vandenberghe, R. Amyloid imaging in cognitively normal individuals, at-risk populations and preclinical Alzheimer’s disease. Neuroimage Clin. 2013, 2, 356–365.
- Jack, C.R., Jr.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish, J.; et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimer’s Dement. 2018, 14, 535–562.
- Tosun, D.; Joshi, S.; Weiner, M.W.; Alzheimer’s Disease Neuroimaging Initiative. Neuroimaging predictors of brain amyloidosis in mild cognitive impairment. Ann. Neurol. 2013, 74, 188–198.
- Lee, J.H.; Byun, M.S.; Yi, D.; Sohn, B.K.; Jeon, S.Y.; Lee, Y.; Lee, J.Y.; Kim, Y.K.; Lee, Y.S.; Lee, D.Y. Prediction of Cerebral Amyloid With Common Information Obtained From Memory Clinic Practice. Front. Aging Neurosci. 2018, 10, 309.
- Shan, G.; Bernick, C.; Caldwell, J.Z.K.; Ritter, A. Machine learning methods to predict amyloid positivity using domain scores from cognitive tests. Sci. Rep. 2021, 11, 4822.
- Mielke, M.M.; Wiste, H.J.; Weigand, S.D.; Knopman, D.S.; Lowe, V.J.; Roberts, R.O.; Geda, Y.E.; Swenson-Dravis, D.M.; Boeve, B.F.; Senjem, M.L.; et al. Indicators of amyloid burden in a population-based study of cognitively normal elderly. Neurology 2012, 79, 1570–1577.
- Ansart, M.; Epelbaum, S.; Gagliardi, G.; Colliot, O.; Dormont, D.; Dubois, B.; Hampel, H.; Durrleman, S.; Alzheimer’s Disease Neuroimaging Initiative; INSIGHT-preAD Study. Reduction of recruitment costs in preclinical AD trials: Validation of automatic pre-screening algorithm for brain amyloidosis. Stat. Methods Med. Res. 2020, 29, 151–164.
- Jack, C.R., Jr.; Knopman, D.S.; Jagust, W.J.; Shaw, L.M.; Aisen, P.S.; Weiner, M.W.; Petersen, R.C.; Trojanowski, J.Q. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010, 9, 119–128.
- Mormino, E.C.; Betensky, R.A.; Hedden, T.; Schultz, A.P.; Amariglio, R.E.; Rentz, D.M.; Johnson, K.A.; Sperling, R.A. Synergistic effect of beta-amyloid and neurodegeneration on cognitive decline in clinically normal individuals. JAMA Neurol. 2014, 71, 1379–1385.
- Tong, T.; Gao, Q.; Guerrero, R.; Ledig, C.; Chen, L.; Rueckert, D.; Initiative, A.D.N. A Novel Grading Biomarker for the Prediction of Conversion From Mild Cognitive Impairment to Alzheimer’s Disease. IEEE Trans. Biomed. Eng. 2017, 64, 155–165.
- Cummings, J. The National Institute on Aging-Alzheimer’s Association Framework on Alzheimer’s disease: Application to clinical trials. Alzheimer’s Dement. 2019, 15, 172–178.
- Allison, S.L.; Koscik, R.L.; Cary, R.P.; Jonaitis, E.M.; Rowley, H.A.; Chin, N.A.; Zetterberg, H.; Blennow, K.; Carlsson, C.M.; Asthana, S.; et al. Comparison of different MRI-based morphometric estimates for defining neurodegeneration across the Alzheimer’s disease continuum. Neuroimage Clin. 2019, 23, 101895.
- Young, P.N.E.; Estarellas, M.; Coomans, E.; Srikrishna, M.; Beaumont, H.; Maass, A.; Venkataraman, A.V.; Lissaman, R.; Jimenez, D.; Betts, M.J.; et al. Imaging biomarkers in neurodegeneration: Current and future practices. Alzheimer’s Res. 2020, 12, 49.
- Farhan, S.; Fahiem, M.A.; Tauseef, H. An ensemble-of-classifiers based approach for early diagnosis of Alzheimer’s disease: Classification using structural features of brain images. Comput. Math. Methods Med. 2014, 2014, 862307.
- Ahmed, O.B.; Mizotin, M.; Benois-Pineau, J.; Allard, M.; Catheline, G.; Ben Amar, C.; Alzheimer’s Disease Neuroimaging Initiative. Alzheimer’s disease diagnosis on structural MR images using circular harmonic functions descriptors on hippocampus and posterior cingulate cortex. Comput. Med. Imaging Graph. 2015, 44, 13–25.
- Korolev, S.; Safiullin, A.; Belyaev, M.; Dodonova, Y. Residual and plain convolutional neural networks for 3D brain MRI classification. In Proceedings of the 2017 IEEE 14th International Symposium on Biomedical Imaging (ISBI 2017), Melbourne, Australia, 18–21 April 2017; pp. 835–838.
- Costafreda, S.G.; Dinov, I.D.; Tu, Z.; Shi, Y.; Liu, C.Y.; Kloszewska, I.; Mecocci, P.; Soininen, H.; Tsolaki, M.; Vellas, B.; et al. Automated hippocampal shape analysis predicts the onset of dementia in mild cognitive impairment. Neuroimage 2011, 56, 212–219.
- Lebedev, A.V.; Westman, E.; Van Westen, G.J.; Kramberger, M.G.; Lundervold, A.; Aarsland, D.; Soininen, H.; Kloszewska, I.; Mecocci, P.; Tsolaki, M.; et al. Random Forest ensembles for detection and prediction of Alzheimer’s disease with a good between-cohort robustness. Neuroimage Clin. 2014, 6, 115–125.
- Spasov, S.; Passamonti, L.; Duggento, A.; Lio, P.; Toschi, N.; Alzheimer’s Disease Neuroimaging Initiative. A parameter-efficient deep learning approach to predict conversion from mild cognitive impairment to Alzheimer’s disease. Neuroimage 2019, 189, 276–287.
- Doody, R.S.; Massman, P.; Dunn, J.K. A method for estimating progression rates in Alzheimer disease. Arch. Neurol. 2001, 58, 449–454.
- Nagahama, Y.; Nabatame, H.; Okina, T.; Yamauchi, H.; Narita, M.; Fujimoto, N.; Murakami, M.; Fukuyama, H.; Matsuda, M. Cerebral correlates of the progression rate of the cognitive decline in probable Alzheimer’s disease. Eur. Neurol. 2003, 50, 1–9.
- Barocco, F.; Spallazzi, M.; Concari, L.; Gardini, S.; Pelosi, A.; Caffarra, P. The Progression of Alzheimer’s Disease: Are Fast Decliners Really Fast? A Four-Year Follow-Up. J. Alzheimer’s Dis. 2017, 57, 775–786.
- Edwin, T.H.; Strand, B.H.; Persson, K.; Engedal, K.; Selbaek, G.; Knapskog, A.B. Trajectories and risk factors of dementia progression: A memory clinic cohort followed up to 3 years from diagnosis. Int. Psychogeriatr. 2021, 33, 779–789.
- Deaton, A.; Cartwright, N. Understanding and misunderstanding randomized controlled trials. Soc. Sci. Med. 2018, 210, 2–21.
- Jutten, R.J.; Sikkes, S.A.M.; Van der Flier, W.M.; Scheltens, P.; Visser, P.J.; Tijms, B.M.; Alzheimer’s Disease Neuroimaging Initiative. Finding Treatment Effects in Alzheimer Trials in the Face of Disease Progression Heterogeneity. Neurology 2021, 96, e2673–e2684.
- Cummings, J.L.; Morstorf, T.; Zhong, K. Alzheimer’s disease drug-development pipeline: Few candidates, frequent failures. Alzheimer’s Res. 2014, 6, 37.
- Tolar, M.; Abushakra, S.; Hey, J.A.; Porsteinsson, A.; Sabbagh, M. Aducanumab, gantenerumab, BAN2401, and ALZ-801-the first wave of amyloid-targeting drugs for Alzheimer’s disease with potential for near term approval. Alzheimer’s Res. 2020, 12, 95.
- Langford, O.; Raman, R.; Sperling, R.A.; Cummings, J.; Sun, C.K.; Jimenez-Maggiora, G.; Aisen, P.S.; Donohue, M.C. Predicting Amyloid Burden to Accelerate Recruitment of Secondary Prevention Clinical Trials. J. Prev. Alzheimer’s Dis. 2020, 7, 213–218.
- Zhou, Y.; Wang, F.; Tang, J.; Nussinov, R.; Cheng, F. Artificial intelligence in COVID-19 drug repurposing. Lancet Digit. Health 2020, 2, e667–e676.
- Ying, R.; Bourgeois, D.; You, J.; Zitnik, M.; Leskovec, J. Gnnexplainer: Generating explanations for graph neural networks. Adv. Neural Inf. Process. Syst. 2019, 32, 9240.
