Successful oncologic resection in rectal cancer relies on the surgical tenants of wide local excision of the disease to establish clear margin status and resection of adequate lymph nodes for staging. The introduction of, and adherence to, complete total mesorectal excision, which is the resection of the total mesorectal envelope containing the local lymphatic drainage of the rectum, has contributed a large part to the improved operative outcomes and long-term survival for rectal cancer patients. Notably, total mesorectal excision has produced a notable reduction in rectal cancer recurrence by 30 to 40%.
- Surgical
- Rectal Cancer
- Transanal Total Mesorectal Excision
1. Introduction
2. Robotic Total Mesorectal Excision
3. Transanal Total Mesorectal Excision
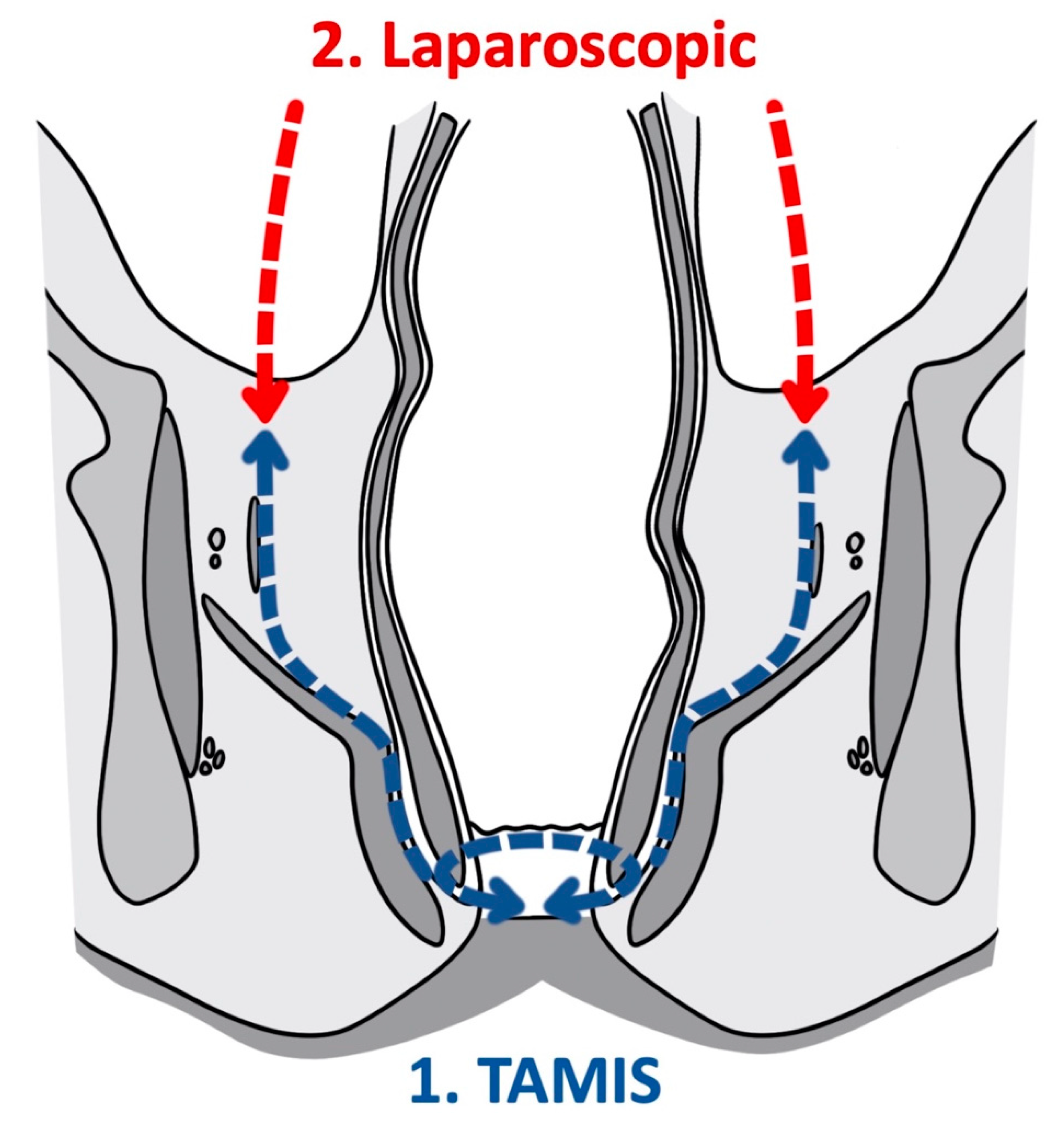
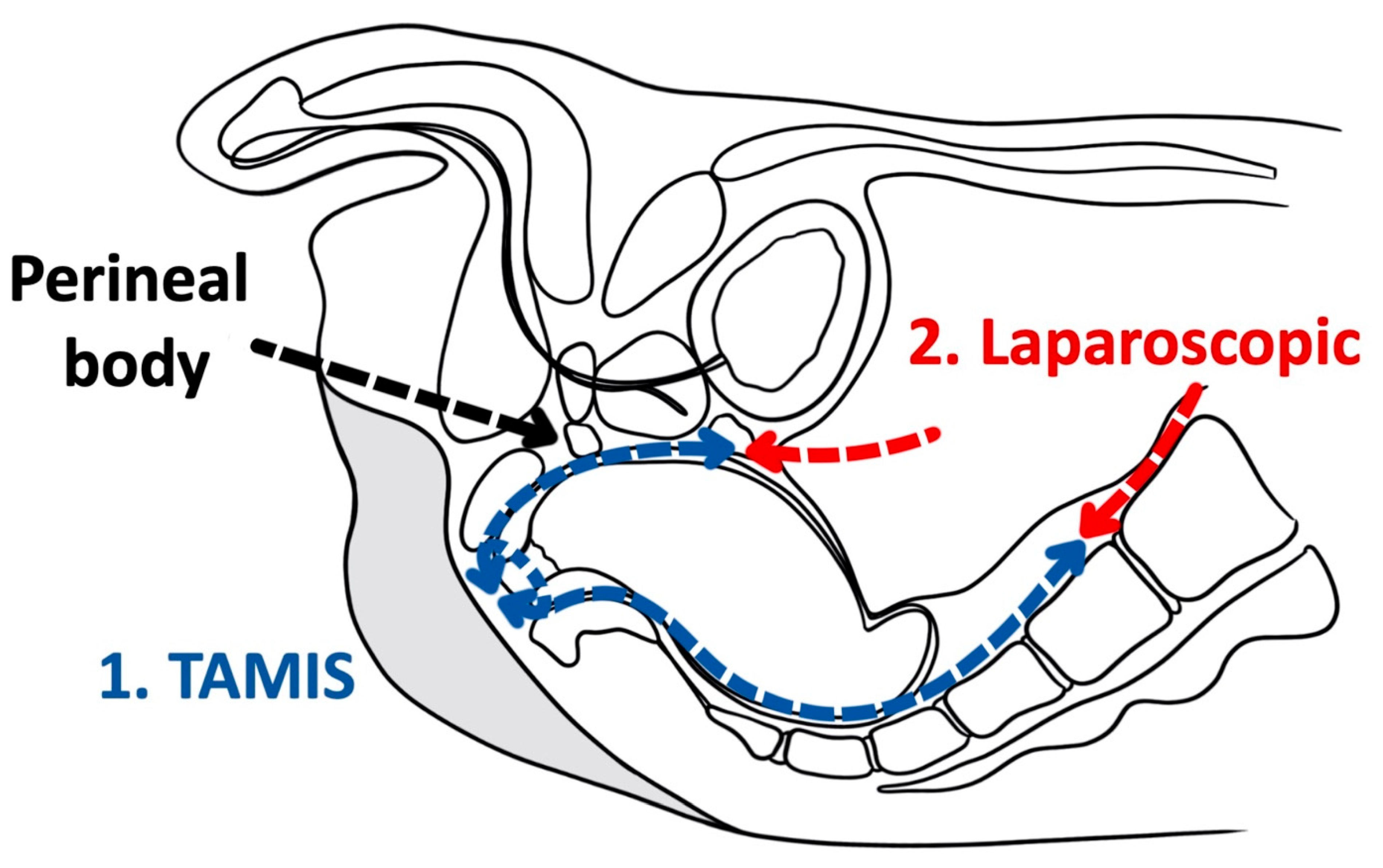
This entry is adapted from the peer-reviewed paper 10.3390/cancers14040938
References
- Parfitt, J.R.; Driman, D.K. The total mesorectal excision specimen for rectal cancer: A review of its pathological assessment. J. Clin. Pathol. 2007, 60, 849–855.
- Quirke, P.; Steele, R.; Monson, J.; Grieve, R.; Khanna, S.; Couture, J.; O’Callaghan, C.; Myint, A.S.; Bessell, E.; Thompson, L.C.; et al. Effect of the plane of surgery achieved on local recurrence in patients with operable rectal cancer: A prospective study using data from the MRC CR07 and NCIC-CTG CO16 randomised clinical trial. Lancet 2009, 373, 821–828.
- Bujko, K.; Rutkowski, A.; Chang, G.J.; Michalski, W.; Chmielik, E.; Kusnierz, J. Is the 1-cm rule of distal bowel resection margin in rectal cancer based on clinical evidence? A systematic review. Ann. Surg. Oncol. 2012, 19, 801–808.
- Kuvshinoff, B.; Maghfoor, I.; Miedema, B.; Bryer, M.; Westgate, S.; Wilkes, J.; Ota, D. Distal margin requirements after preoperative chemoradiotherapy for distal rectal carcinomas: Are ≤ 1 cm Distal Margins Sufficient. Ann. Surg. Oncol. 2001, 8, 163–169.
- Andreola, S.; Leo, E.; Belli, F.; Lavarino, C.; Bufalino, R.; Tomasic, G.; Baldini, M.T.; Valvo, F.; Navarria, P.; Lombardi, F. Distal intramural spread in adenocarcinoma of the lower third of the rectum treated with total rectal resection and coloanal anastomosis. Dis. Colon Rectum 1997, 40, 25–29.
- Nguyen, T.H.; Chokshi, R.V. Low Anterior Resection Syndrome. Curr. Gastroenterol. Rep. 2020, 22, 48.
- Abraham, N.S.; Young, J.M.; Solomon, M.J. Meta-analysis of short-term outcomes after laparoscopic resection for colorectal cancer. Br. J. Surg. 2004, 91, 1111–1124.
- Heikkinen, T.; Msika, S.; Desvignes, G.; Schwandner, O.; Schiedeck, T.H.; Shekarriz, H.; Bloechle, C.H.; Baca, I.; Weiss, O.; Morino, M.; et al. Laparoscopic surgery versus open surgery for colon cancer: Short-term outcomes of a randomised trial. Lancet Oncol. 2005, 6, 477–484.
- Fleshman, J.; Sargent, D.J.; Green, E.; Anvari, M.; Stryker, S.J.; Beart, R.W.; Hellinger, M.; Flanagan, R.; Peters, W.; Nelson, H. Laparoscopic colectomy for cancer is not inferior to open surgery based on 5-year data from the COST Study Group trial. Ann. Surg. 2007, 246, 655–662.
- Jayne, D.G.; Guillou, P.J.; Thorpe, H.; Quirke, P.; Copeland, J.; Smith, A.M.H.; Heath, R.M.; Brown, J.M. Randomized trial of laparoscopic-assisted resection of colorectal carcinoma: 3-Year results of the UK MRC CLASICC trial group. J. Clin. Oncol. 2007, 25, 3061–3068.
- Jayne, D.; Pigazzi, A.; Marshall, H.; Croft, J.; Corrigan, N.; Copeland, J.; Quirke, P.; West, N.; Rautio, T.; Thomassen, N.; et al. Effect of robotic-assisted vs conventional laparoscopic surgery on risk of conversion to open laparotomy among patients undergoing resection for rectal cancer the rolarr randomized clinical trial. JAMA J. Am. Med. Assoc. 2017, 318, 1569–1580.
- Rouanet, P.; Bertrand, M.M.; Jarlier, M.; Mourregot, A.; Traore, D.; Taoum, C.; de Forges, H.; Colombo, P.E. Robotic Versus Laparoscopic Total Mesorectal Excision for Sphincter-Saving Surgery: Results of a Single-Center Series of 400 Consecutive Patients and Perspectives. Ann. Surg. Oncol. 2018, 25, 3572–3579.
- Kim, M.J.; Park, S.C.; Park, J.W.; Chang, H.J.; Kim, D.Y.; Nam, B.H.; Sohn, D.K.; Oh, J.H. Robot-assisted Versus Laparoscopic Surgery for Rectal Cancer: A Phase II Open Label Prospective Randomized Controlled Trial. Ann. Surg. 2018, 267, 243–251.
- Gavriilidis, P.; Wheeler, J.; Spinelli, A.; de‘Angelis, N.; Simopoulos, C.; Di Saverio, S. Robotic vs laparoscopic total mesorectal excision for rectal cancers: Has a paradigm change occurred? A systematic review by updated meta-analysis. Colorectal Dis. 2020, 22, 1506–1517.
- Jeon, Y.; Park, E.J.; Baik, S.H. Robotic Surgery for Rectal Cancer and Cost-Effectiveness. J. Minim. Invasive Surg. 2019, 22, 139–149.
- Yamaoka, Y.; Yamaguchi, T.; Kinugasa, Y.; Shiomi, A.; Kagawa, H.; Yamakawa, Y.; Furutani, A.; Manabe, S.; Torii, K.; Koido, K.; et al. Mesorectal fat area as a useful predictor of the difficulty of robotic-assisted laparoscopic total mesorectal excision for rectal cancer. Surg. Endosc. 2019, 33, 557–566.
- Escal, L.; Nougaret, S.; Guiu, B.; Bertrand, M.M.; de Forges, H.; Tetreau, R.; Thézenas, S.; Rouanet, P. MRI-based score to predict surgical difficulty in patients with rectal cancer. Br. J. Surg. 2018, 105, 140–146.
- Cavaliere, R.; Tedesco, M.; Giannarelli, D.; Aloe, L.; Perri, P.; Di Filippo, F.; Crecco, M.; Gabrielli, F.; Cosimelli, M.; Stipa, S. Radical surgery in rectal cancer patients: What does it mean today? J. Surg. Oncol. 1991, 48, 24–31.
- Sylla, P.; Rattner, D.W.; Delgado, S.; Lacy, A.M. NOTES transanal rectal cancer resection using transanal endoscopic microsurgery and laparoscopic assistance. Surg. Endosc. 2010, 24, 1205–1210.
- De Lacy, A.M.; Rattner, D.W.; Adelsdorfer, C.; Tasende, M.M.; Fernández, M.; Delgado, S.; Sylla, P.; Martínez-Palli, G. Transanal natural orifice transluminal endoscopic surgery (NOTES) rectal resection: “Down-to-up” total mesorectal excision (TME)—Short-term outcomes in the first 20 cases. Surg. Endosc. 2013, 27, 3165–3172.
- Veltcamp Helbach, M.; Deijen, C.L.; Velthuis, S.; Bonjer, H.J.; Tuynman, J.B.; Sietses, C. Transanal total mesorectal excision for rectal carcinoma: Short-term outcomes and experience after 80 cases. Surg. Endosc. 2016, 30, 464–470.
- Tuech, J.J.; Karoui, M.; Lelong, B.; De Chaisemartin, C.; Bridoux, V.; Manceau, G.; Delpero, J.R.; Hanoun, L.; Michot, F. A step toward notes total mesorectal excision for rectal cancer endoscopic transanal proctectomy. Ann. Surg. 2015, 261, 228–233.
- Lacy, A.M.; Tasende, M.M.; Delgado, S.; Fernandez-Hevia, M.; Jimenez, M.; De Lacy, B.; Castells, A.; Bravo, R.; Wexner, S.D.; Heald, R.J. Transanal Total Mesorectal Excision for Rectal Cancer: Outcomes after 140 Patients. J. Am. Coll. Surg. 2015, 221, 415–423.
- Denost, Q.; Adam, J.P.; Rullier, A.; Buscail, E.; Laurent, C.; Rullier, E. Perineal transanal approach: A new standard for laparoscopic sphincter-saving resection in low rectal cancer, a randomized trial. Ann. Surg. 2014, 260, 993–999.
- Deijen, C.L.; Velthuis, S.; Tsai, A.; Mavroveli, S.; de Lange-de Klerk, E.S.M.; Sietses, C.; Tuynman, J.B.; Lacy, A.M.; Hanna, G.B.; Bonjer, H.J. COLOR III: A multicentre randomised clinical trial comparing transanal TME versus laparoscopic TME for mid and low rectal cancer. Surg. Endosc. 2016, 30, 3210–3215.
