Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Immunology
Exosomes are membrane-enveloped nanosized (30–150 nm) extracellular vesicles of endosomal origin produced by almost all cell types and encompass a multitude of functioning biomolecules. Exosomes have been considered crucial players of cell-to-cell communication in physiological and pathological conditions. Accumulating evidence suggests that exosomes can modulate the immune system by delivering a plethora of signals that can either stimulate or suppress immune responses, which have potential applications as immunotherapies for cancer and autoimmune diseases.
- exosomes
- immunotherapy
- extracellular vesicles
1. Introduction
Exosomes are small endosomal origin extracellular vesicles (EVs) with a lipid bilayer, 30–150 nm, secreted by almost all cell types in the extracellular space such as blood, and travel to distant tissues [3,4,5]. According to the recently updated guidelines of the International Society for Extracellular Vesicles (ISEV) on minimal information for studies of extracellular vesicles (MISEV), the term “extracellular vesicle” or “EV” has now been agreed on as the consensus generic term for lipid bilayer-delimited particles released from the cell and cannot replicate [6]. They carry various biologically active macromolecules such as proteins, RNA, DNA, miRNAs and metabolites, which act as mediators in cell-to-cell communication [7,8,9]. Exosomes are formed by endocytosis of the endosome. In the process, there is endocytosis and scission of invaginated plasma membrane leading to the formation of endosomes. With the help of the Golgi complex, these newly formed vesicles turn from early endosomes to late endosomes [10,11,12,13]. The resulting endosome creates intraluminal vesicles (ILVs) in the lumen of the endosome through several invaginations of the membrane [14]. This process of invagination of the endosomal membrane in the lumen is controlled by endosomal sorting complexes required for transport (ESCRT) [15]. These proteins assist in sorting endosomal proteins and cell contents in ILVs, leading to the formation of mature endosomes called multivesicular bodies (MVBs) [16,17]. These MVBs then fuse with the cell membrane and later release their internal vesicles, i.e., exosomes, in extracellular space. Exosomes are differentiated based on their size, exosomal markers, RNA, and other special proteins [18].
The exosomes are involved in multiple different pathways affecting multiple systems. Research has shown their involvement in activating the immune system through different pathways. They include increasing cell activity and enhancing the release of natural killer cells, tumor necrosis cells, inducing macrophages [19,20,21,22,23]. Furthermore, they also play a role in signal transfer among numerous types of neurons, although the mechanism is still unclear [24,25]. They are also heavily involved in cell proliferation by carrying contents from parental cells and acting as stem cells mediators.
2. Isolation, Purification and Characterization of Exosomes
Exosomes have been isolated from a variety of bodily fluids including bile, blood, breast milk, urine, cerebrospinal fluid, and saliva as well as from in vitro culture media [26]. Although exosomes have enormous prospects for clinical use as therapeutic, diagnostic, and prognostic probes, the progress is somewhat subdued by our inadequate knowledge of the most efficient and reproducible approach for large-scale production of quality exo-somes in a short time. Several isolation techniques have been developed by exploiting a particular trait, such as the size, density and surface markers of exosomes which includes ultracentrifugation, density gradient centrifugation, ultrafiltration, an immunoaffinity capture-based method, microfluidics-based techniques and a polyethylene glycol (PEG)-based isolation method [27,28,29]. Up to the present, the most widely adopted and reliable method is ultracentrifugation, which involves a series of centrifugation steps to remove cells, large vesicles, debris and precipitate exosomes. But ultracentrifugation method is tedious, time-consuming that needs special equipment and unable to separate impurities like viruses, apoptotic bodies and proteins.
3. Biomolecular Components of Exosomes in Immunomodulation and Exosome-Mediated Regulation of Immune Cells
Biomolecular components of exosomes play crucial role in modulating immune system and regulating functions of immune cells. A summary of the mechanisms and effects of these molecules can be found in Figure 1.
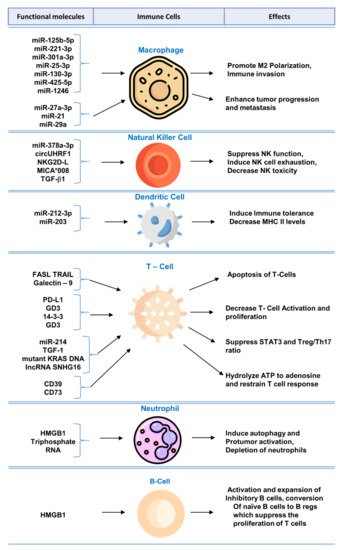
Figure 1. Functional molecules in tumor-derived exosomes for immunomodulation and exosome-mediated regulation of immune cells.
3.1. Exosomal Biomolecules Modulating T-Cell Function
Tumor-derived exosomes express multiple functional biomolecules that have been shown to impair T lymphocyte function, such as by induction of T cell apoptosis [38], inhibition of activation [46], or by suppression of function [44,45].
Oral squamous cell carcinoma-derived exosomes expressing FasL have been studied to show induction of apoptosis of T cells via receptor and mitochondrial pathways [40]. FasL-bearing exosomes also correlated with poor prognosis and nodal involvement [40]. Prostatic cancer cell line-derived exosomes carrying FasL showed dose-dependent apoptosis of CD8+ T cells and inhibited T cell proliferation in co-culture [38]. The addition of anti-FasL antibody prevented the apoptosis of CD8+ T cells by tumor-derived exosomes [38].
3.2. Exosomal Biomolecules Modulating NK Cell Function
NK cells are an important component of the innate immune system; they identify MHC-I-lacking tumor cells, bind to stress-induced ligands on tumor cells, and become activated to kill tumor cells [72]. Tumor-derived exosomes have been shown to stimulate [21] or inhibit [57] the activity of NK cells. Inhibition of NK cell activation via tumor-derived exosomes leads to the escape of tumor cells from NK cell immune surveillance [59]. Tumor-derived exosomes have been shown to inhibit NK cells by blocking their activation via IL-2 [73]. Tumor-derived exosomes carrying MICA*008 molecules target NK cells and downregulate surface NKG2D receptors and minimize NK cytotoxicity leading to immune evasion [54]. Mesothelioma-derived exosomes expressing TGFβ1 and NKG2DL impair NK cell activation by downregulating surface NKG2D on NK cells [57].
3.3. Exosomal Biomolecules Responsible for the Polarization of Macrophages
HCC derived exosomes, expressing miR-146-5p, lead to formation of M2-polarized tumor-associated macrophages, which inhibited the expression of IFN-γ and TNF-α while upregulating inhibitory receptors of such as PD-1 and CTLA-4 in T cells resulting in T cell exhaustion. Transcription factor Sal-like protein-4 (SALL4) was essential for regulating miR-146a-5p in HCC exosomes. Blocking the SALL4/miR-146a-5p interaction delayed HCC progression in mice model [51]. Polarization of M0 macrophage to M2 macrophage via exosomes released from tumor cells has also been observed in lung cancer [75].
3.4. Exosomal Biomolecules Modulating B-Cell Function
Studies have shown that exosomes play a role in altering B cell function [76]. Exosomes derived from HCC, exhibiting high mobility group box-1 (HMGB1), have been shown to increase proliferation of TIM-1+ regulatory B cells, which expressed IL-10 and suppressed CD8+ T cell proliferation leading to immune tolerance. Exosomal HMGB1 promoted TIM-1+ Breg cell proliferation via Toll-like receptor (TLR) 2/4 and mitogen-activated protein kinase (MAPK) signaling pathways [60]. In the tumor microenvironment, Bregs target tumor-infiltrating immune cells and interrupt antitumor immunity [77].
3.5. Exosomal Biomolecules Modulating Dendritic Cell (DC) Function
Tumor-derived exosomes, expressing IL-6, have been shown to suppress differentiation of bone marrow precursors into immature dendritic cells [61]. Ding G et al. reported that pancreatic tumors shed exosomes expressing miRNA-212-3p, which inhibit RFXAP expression and downregulate MHC-II expression on dendritic cells leading to immune tolerance [62]. In another study on Epstein-Barr virus (EBV)-associated gastric carcinoma (EBVaGC), it has been observed that EBV infected epithelial cells shed exosomes that inhibit dendritic cell maturation [78]. On the other hand, exosomes derived from squamous cell carcinoma, treated by 5-aminolevulinic acid photodynamic therapy, are able to stimulate DC maturation and promote antitumor immune response [79].
3.6. Exosomal Biomolecules Modulating Myeloid-Derived Suppressor Cell (MDSC) Function
Myeloid-derived suppressor cells inhibit T cell response in the tumor microenvironment [85]. It has been reported that gastric cancer cells release exosomes bearing miR-107 that cause activation and expansion of MDSCs [63].
4. Tumor Cell-Derived Exosomes in Immunotherapy
Tumor cells have emerged as a potential source for emitting exosomes [86]. Furthermore, it was found that tumor cells derive more exosomes than normal cells in the human body [87]. Tumor-derived exosomes (TEXs) act as a double-edged sword as they can play important role in cancer progression and metastasis by decreasing cytotoxicity and maintaining immunosuppressive tumor microenvironment as well as can improvise anti-tumor immune response by carrying tumor antigens and several heat shock proteins (HSPs) such as HSP70, HSP90 [88].
5. Immune Cell-Derived Exosomes in Immunotherapy
The immune system has an innate and an adaptive constituent, each having a distinct role and function. There are a variety of cells that take part in the innate and adaptive immunity. Recent studies have demonstrated exosomes are involved in the intercellular communication in the immune system as well as playing a role in immune modulation [101,102]. Exosomes released from immune cells can contain molecules that are essential for immune response initiation, antigen presentation along with intercellular exchange of membrane or cytosolic components without even the cell being in proximity to each other [103]. Moreover, based on the proteins packed in the exosomes, their target receptors and type of immune cell activation can vary (Figure 2). Recent studies have demonstrated antitumor effect of DC-derived tumor cells on top of their role in immunity [104,105].
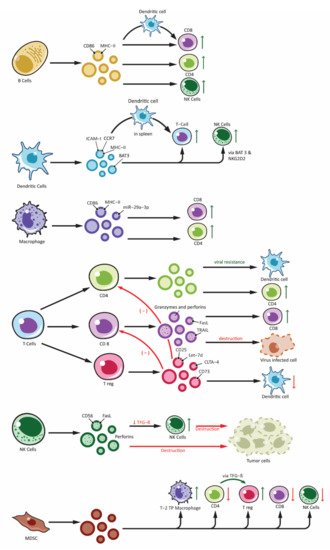
Figure 2. Effects of immune cell-derived exosomes on cellular components of immune system. Showing exosomes produced from various immune cells: B-lymphocytes (B cells), T-lymphocytes (T Cells along with subdivisions of CD4+, CD8+ and Treg), Natural Killer cells (NK-cells), Dendritic cells and other antigen presenting cells (APCs) and Myeloid Derived Suppressor Cells (MDSC). Immune cell derived exosomes can affect various other cells participating in the immune responses. Exosomes can stimulate immune response by: peptide-MHC complex presentation to T cells, antigen transfer to other dendritic cells, activation of T cells, B cells and NK cells. Similarly, inhibitory function is derived from exosomes from Treg and NK cells and this include overall inhibition of the adaptive arm of immune response as well as destruction of tumor cells by NK cell. Exosomes from MDSC also plays an important role in immune inhibition mainly by activating Treg and Type-2 Tumor promoting phenotype macrophages (T-2 TP macrophage).
6. Modulation and Evasion of the Complement System by Exosomes
Often regarded as the first line of defense against pathogens, the complement system is an important component of innate immunity [159]. Not only does it play an important role in opsonization and elimination of pathogens, activation of complement cascade results in the release of chemokines and cytokines, interact with coagulation cascade and regulate inflammation process and repair [160]. The complement system consists of more than 30 proteins and activated in one of three pathways, classic pathway, alternate pathway and lectin pathway [161]. Each of the three pathway results in the generation of C3 convertase which subsequently triggers formation of membrane attack complex (MAC) and cell lysis [162]. During local and systemic inflammation, there are more circulating exosomes, as well as elevated complement activation products, this suggests a link between exosome and complement system [163]. Exosomes has been shown to play a role in complement activation via different molecules on their surface (Figure 3). Exosomes released from APCs are likely to be loaded with antigenic protein and hence have the potential to bind to immunoglobulins and thereafter cause activation of classic pathway [164].
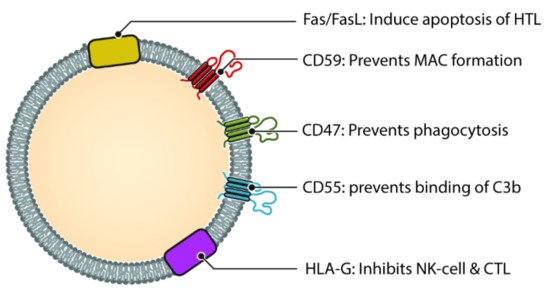
Figure 3. Different molecules that help exosomes escaping complement and immune system. Helper T cell (HTL), Natural Killer cell (NK Cell) and Cytotoxic T-lymphocyte (CTL). CD47, CD55 and CD59 help prevent the exosome from being destroyed by the complement cascade, either directly (via MAC) or indirectly. Molecules such as HLA-G and Fas/FasL protect the exosome from immune mediated destruction via cell mediated immunity (Cytotoxic T cell and NK cell).
7. Engineered Exosomes in Immunotherapy
Exosomes have opened a new direction for treating life-threatening diseases by immunotherapy which is further strengthened by various engineering mechanisms to trigger a stronger immune response. Not only biomimetic but also artificial exosomes can be used for immunotherapy. Biomimetic exosomes can deliver monoclonal antibody DEC205 to DCs [182]. Artificial exosomes are utilized for targeting DCs and activating T cells by modifying them with MHC-1 peptides and liposomes; liposome-containing exosomes presented to be stable and appropriate as well [183].
7.1. Genetic Engineering
Several mechanisms of genetic engineering have been applied to modify the exosomes to elicit better outcomes in immunotherapy. They are proven to be a suitable delivery vehicle for antibodies as well as chemotherapeutic drugs based on their promising modifiability, compatibility and cyclical half-life [184].
7.2. miRNA Modification
Exosomes treated with DHA (docosahexaenoic acid) have been found to express increased levels of let-7a, miR-23b, miR-27a/b, miR-21, let-7, and miR-320b and these are tumor suppressor miRNA. When these treated exosomes are incubated with unexposed epithelial cells, the epithelial cells also express higher levels of tumor-suppressing miRNA. Consequently, exosome-mediated signaling between tumor cells should be targeted for immunotherapy [198].
7.3. Conjugation
The process of conjugation is used to bind different activator molecules with exosomes to stimulate and activate the immune cells. TLR ligands such as CpG-DNA, Poly:IC and IL15Rα conjugated to TEXs can enhance immune response. NKG2D ligand binding can also activate natural killer cells [203]. Chondroitin sulfate (CS) is increasingly expressed in cancer cells. VAR2CSA is a malarial protein that binds to VAR2CSA-ligand or CS which is expressed in placental tissue. Therefore, CS in malignant cells could be potentially targeted by recombinant VAR2CSA (rVAR2). Targeting the common CS chain which is present on various cancer cells could offer a novel treatment pathway [204]. A new strategy named exosomes for protein loading via optically reversible protein-protein interactions (EXPLORs) where blue light controls the integration of protein-protein interaction during endogenous exosome biogenesis. This method is used for intracellular delivery of target proteins [205].
7.4. HSP70/90 Overexpression (Heat Treatment)
HSP expressed on exosomes are recognized as a potential target for immunotherapy. Different HSP have been identified on the surface of TEXs such as 71-kDa HSP, the 70-kDa HSP4, and HSP90 alpha and beta [134]. HSP70 expressing exosomes derived from pancreatic carcinoma cells enhance lytic activity of natural killer cells against HSP70 surface positive tumors [21].
8. Advantages of Using Exosomes for Immunotherapy
Structurally, exosome possesses an intermediate level of organization that distinguishes them from other molecular or organ levels of therapeutics. They contain heterogeneous functional molecules as well as avoid the complexity of organ-level [90]. Also, the gel-like cytoplasm and deformable cytoskeleton provide structural integrity and rupture resistance that makes exosome an exclusive diagnostic, prognostic, or therapeutic tool [210]. Natural cargos like lipids, proteins, nucleic acids are used for these purposes. These molecules can also be modified according to specific use. The modification strategies include adapting surface molecules to enable specific targeting, passive loading of hydrophobic drugs on membrane & introducing hydrophilic cargos into the core [210].
Being comprised with lipid-bilayer, exosomes effectively transfer their cargo without any interference by extra-cellular enzymes & maintain their bioavailability. Most of the drugs have poor oral bioavailability as they have to encounter the first-pass metabolism by the liver. So, these drugs are introduced intravenously to retain their bioavailability [211]. But concerning the convenience of oral administration over IV administration, cow milk exosomes have demonstrated the potential for boosting oral drug bioavailability in several clinical trials [212,213,214].
Exosomes play a key role in the detection of various diseases as they contain specific biomarkers (proteins, lipids, RNAs) in their lumen. They possess ’clathrin-coat’ on their membrane which protects their content from degradation by enzymes such as RNases. This coat makes them more stable, resistant & enables them to transport various components efficiently (drug delivery) [36,215,216,217]. So, they have been significantly used as a diagnostic tool to detect lung cancer, Alzheimer’s disease, colon cancer & many other diseases [218,219,220]. Also, they can be used as non-invasive biomarkers as they are found in several body fluids like plasma, saliva, urine, gingival crevicular fluid, etc. [221].
9. Conclusions
Because of its specificity and fewer side effects compared to the other modality of therapy, immunotherapy emerged as one of the most explored areas in the new era of numerous diseases, specifically in cancer treatment. To develop targeted immunotherapy, it is indispensable to understand the mechanism of immune responses during the disease process and reciprocal interaction between the pathological tissue and cellular components of the immune system. In this regard, accumulating evidence suggests that exosomes play a crucial role in intercellular communication and depending on the parent cell type and the cargo, exosomes can have immunosuppressive or immunostimulatory effects. Due to their intrinsic properties and advantages, exosomes can be utilized as immunotherapeutics and as a carrier for other immunomodulatory agents. Although there are some limitations in isolation and clinical application, exosome-based immunotherapies have immense potential which can revolutionize the treatment of cancer and other pathologies. Hence, this is a compelling area of research that needs extensive exploration to comprehend the role of exosomes in disease pathology and immune modulation and to use these concepts for the development of new exosome-based immunotherapies.
This entry is adapted from the peer-reviewed paper 10.3390/jnt3010005
This entry is offline, you can click here to edit this entry!
