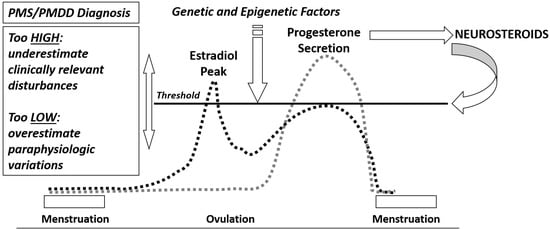
Premenstrual symptoms are very common, affecting about half of women in reproductive age worldwide
[9]. However, prevalence rates vary widely in different studies and countries depending on samples, methods of investigation and diagnostic criteria. Disparities may also derive from genetic and socio-cultural factors, including diet and life-style, stressors, personal attitudes, coping behaviors, workload and family responsibilities
[9]. Available surveys in community populations indicate that PMS affects 20–30% of women, whereas PMDD ranges between 1.2 and 6.4%
[10], with black women being significantly less likely to experience PMDD and PMS than white women (odds ratio (OR) 0.44, 95% confidence interval (CI) 0.25–0.79 and OR 0.64, 95% CI 0.47–0.88, respectively), similarly to what is observed in other mental health disorders
[11]. Both conditions significantly reduce quality of life and raise societal costs associated with decreased work productivity, work absenteeism and increased use of health care services
[12]. Prevalence and impact of PMS/PMDD are strong priorities to implement preventive strategies in young women
[13]. Health care providers (HCPs) should be aware that premenstrual symptoms might fluctuate over time with no clear impact of age or reproductive stage, apart from menopausal transition
[14][15]. Another relevant factor is that combined oral contraceptives (COCs), the most studied type of combined hormonal contraception (CHC), may improve overall premenstrual symptomatology in women with PMS/PMDD, but not premenstrual depressive symptoms
[16]. Behavioral risk factors, especially smoking and adiposity, are overrepresented in women with PMS/PMDD, confirming their link to emotional vulnerability. Indeed, smoking was associated with an increased risk of premenstrual disorders (OR = 1.56 (95% CI: 1.25–1.93)). Stratified by diagnosis, the effect size estimate was higher for PMDD (OR = 3.15 (95% CI: 2.20–4.52)) than for PMS (OR = 1.27 (95% CI: 1.16–1.39))
[17]. A strong linear relationship between body mass index (BMI) at baseline and risk of incident PMS, with each 1 kg/m
2 increase in BMI associated with a significant 3% increase in PMS risk (95% confidence interval (CI) 1.01–1.05), was evident
[18]. In particular, women with BMI ≥ 27.5 kg/m
2 at baseline had significantly higher risks of PMS than women with BMI < 20 kg/m
2, following adjustment for age, smoking, physical activity, and other factors
[18]. Intake of alcohol was associated with a moderate increase in the risk of PMS (OR = 1.45, 95% CI: 1.17 to 1.79), especially heavy drinking (OR = 1.79, 95% CI: 1.39 to 2.32) as compared to no or light drinking
[19]. Studies on the effect of exercise have many methodological biases with some suggesting improvement of premenstrual symptoms
[20]. Other proven risk factors include traumatic events, which greatly increased the odds of developing PMDD at follow-up (OR = 4.2, 95% CI = 1.2 to 12.0). Likewise, a history of anxiety disorder (OR = 2.5, 95% CI = 1.1 to 5.5) and elevated daily conflict scores (OR = 1.6, 95% CI = 1.1 to 2.3) predicted PMDD
[21]. Depression may be strongly comorbid
[22][23], in particular postnatally
[24], and women with PMDD should be considered a high-risk group for suicidality, including increased vulnerabilities for suicidal thoughts, ideation, plans and attempts
[25]. Other comorbidities include eating disorders, mainly bulimia and binge eating
[26], and migraine
[27]. The co-occurrence with pathological manifestations displaying premenstrual exacerbations supports a common neuroendocrine etiology
[2][3]. Medical conditions such as anemia and endocrine disorders (namely thyroid and adrenal dysfunctions and hyperprolactinemia)
[28], as well as chronic pelvic pain, fibromyalgia and any other inflammatory disorders
[29][30], may mimic PMS/PMDD symptoms. HCPs should make a differential diagnosis to establish an individualized treatment plan
[3][8][28].