4. Researches and Findings
Soil salinity is an increasingly severe global problem, as salt hampers plant growth and development and reduces crop yield. In addition to naturally occurring soil salinity, salinization increases due to irrigation practices and climate change [
27]. Therefore, in the present study, we explored the response of the tomato seedlings to three different durations of salt stress. Despite the salt-induced oxidative damage, we found that tomato seedlings adopted several strategies, including antioxidative scavenging of ROS and activation of stress-responsive gene signalling to detoxify oxidative stress.
One of the most common symptoms of plants under a stressful environment, e.g., salt or drought stress, includes leaf yellowing and growth inhibition, as plants serve their energy on survival rather than growth improvement [
28]. Similarly, in the present study, increasing salt stress duration induced significant loss of photosynthetic pigments and markedly affected the morphological indices of the tomato seedlings. Such losses in pigment appear to be caused by the reduced activity of chlorophyll biosynthesis enzymes and increased chlorophyllase activity that degraded the chlorophyll content under salt stress [
29,
30]. In addition, sodium ions could disrupt the uptake of some important cation, i.e., Mg
+2 that is the main part of the chlorophyll molecule, thus impacting the chlorophyll content of the leaves. Consequently, it impaired the growth and biomass accumulation in the roots and leaves of tomato seedlings. Consistent with our results, number of previous studies found the loss of pigment and growth inhibition of plants under salt stress [
7,
31,
32].
The excess accumulation of sodium ions under the salt stress imposes osmotic stress, disrupting the water permeability and causing a disturbance in the water potential and turgidity of the cells [
33]. Consistently, in the present study we also noticed that the values of Ψw and Ψs become more negative, enhancing the corresponding Ψp and osmotic adjustments. Ultimately, the RWC declined significantly. Such reduction in RWC of the cells are the primary indicator of the water stress that limits the water flow to the new cell elongation sites [
13]. Similar results were reported earlier in peach [
34] and tomato [
35].
Moreover, salt stress-induced reduction in the water status of the cell results in stomatal closure, inhibiting the gaseous exchange and photosynthetic ability of the leaves [
36]. Combined with the decreased pigment content, it ultimately impairs the photochemical efficiency of the plants. We consistently observed decreased values of the gas exchange parameter and maximal photochemical efficiency (Fv/Fm) in tomato seedlings, indicating the severe consequences of salt-induced osmotic stress on tomato seedling photosynthetic efficiency [
36,
37]. However, the marked increase in osmotic adjustments in tomato seedlings under salt stress also elucidates that it strives to counter the salinity-imposed osmotic stress by modifying the plant-water relations.
Osmotic regulation is a crucial self-defence mechanism produced by plants under stress. Plants produce high levels of osmoprotectants (e.g., soluble sugars, soluble proteins and proline content) to maintain the osmotic pressure and reduce cell water loss [
38]. Proline and soluble sugars accumulated in response to stressful conditions act as a low molecular weight antioxidant that detoxifies the stress-induced toxicity and contributes to cellular osmotic adjustments [
39]. In previous studies with various abiotic stresses, including salinity, these osmolytes increased significantly in chickpea [
40],
Amaranthus tricolor varieties [
41], grapes [
13] and tomatoes [
42]. In addition, burst production of ROS under salt stress could destabilize the protein metabolism, decreasing the contents of soluble proteins in the plants [
43]. Consistent with these reports, in our study, protein content decreased. At the same time, proline and soluble sugars were increased with increasing salt stress duration, suggesting that tomato plants suffered salt-induced toxicity and regulated their osmolytes to survive the water limitations under salt stress.
The declined growth parameters and disturbed plant-water relations due to salinity stress indicate a need to study the plant cells’ sodium accumulation and minerals contents.
In the present study, the salt stress obviously enhanced the Na
+ accumulation in the roots and leaves of tomato seedlings, disturbing the Na
+/K
+ ratio and ion homeostasis [
44,
45]. It could be due to the activation of the guard cells’ outward rectifying K-channels (GORK), which enhanced the Na
+ ion accumulation by the outflow of K
+ and resulted in declined K
+ levels [
44]. Besides the osmotic stress, the higher sodium accumulation in cells could also alter the uptake of other minerals, causing an imbalance in the contents of macro and micronutrients of the plants [
45,
46,
47,
48]. Similarly, in the present study we noticed a significant decline in the contents of important minerals like P, Mg, Mn and K, which is consistent with the decreased growth and chlorophyll contents of the tomato seedlings under salt stress.
In addition, the significant increase in the contents of heavy metals like Cu and Zn under the salt stress could be attributed to the loss of ion specificity due to excessive salt accumulation in the roots and leaves of tomato seedlings [
49]. Previous studies have reported the increase in Ca
2+ ions as a response to salt stress to regulate plant response against salt stress [
50]. In addition, the wheat genotypes with high salinity tolerance maintained the higher calcium content against salt stress, suggesting the important role of Ca ions in maintaining the ion homeostasis and enzyme activities under salt stress [
49]. Consistently, our results also showed higher Ca content in tomato seedlings which could be a response to enduring the salt stress. Collectively, these results showed that salt stress negatively affected the macro and micronutrients in the tomato seedlings. Similar trends were noticed in previous studies with different plant species [
51,
52,
53,
54].
ROS production, MDA accumulation and electrolyte leakage are the basic biomarkers for oxidative stress under abiotic stresses [
55,
56]. In the present study, salt stress induced the production of excessive O
2•− and H
2O
2. Consequently, the higher oxidative stress caused lipid peroxidation and MDA accumulation leading to cell membrane damage and increased leakage of electrolytes as compared to control plants. These results are similar to those in tomato [
36], grape [
13], and chickpea [
40] and demonstrate the severe consequences of salt stress on the growth and development of plants.
Plants have developed different tools against stress which could regulate redox homeostasis and protect plant cells from oxidative damage by scavenging excessive ROS [
57]. For instance, SOD is one of the most important antioxidant enzymes that carry out the dismutation of superoxide anion (O
2•−) into oxygen (O
2) and comparatively less toxic ROS, i.e., hydrogen peroxide (H
2O
2). Subsequently, the other major antioxidant enzymes like POD, APX and CAT detoxify the H
2O
2 to water [
58,
59,
60]. In the present study, salt stress-induced activities of various enzymatic (SOD, POD, CAT, APX, GR) and non-enzymatic antioxidants (AsA, GSH) in the tomato seedlings. These results are in line with the previous reports in tomato [
30] and other species like
Solanum Lycopersicum [
36],
O. sativa [
61],
C. arietinum [
30],
B. juncea [
62,
63] and wheat [
64]. It suggested that tomato seedlings retained the capacity to tolerate or detoxify the salt-induced toxicity for their survival. Another important antioxidants-mediated protective mechanism in plants includes AsA-GSH cycle that assists the antioxidant enzymes to scavenge the excessive ROS. Both AsA and GSH are potent antioxidants and are considered the buffering agents in redox reactions to protect the plasma membrane from stress-induced oxidation [
65]. In this perspective, the H
2O
2 produced after superoxide dismutation is detoxified by APX into H
2O using AsA as the substrate. In the present study, the increased GR activity after salt stress provides GSH, reducing DHAR to dehydroascorbate (DHA) and then to AsA via the AsA–GSH cycle. Similarly, GSH is oxidized to GSSG and subsequently recycled by GR. Thus, the ratio of GSH/GSSG is essential for sustaining the cell redox state [
66]. The increase in the activities of SOD, POD, CAT, APX, GR, MDHAR, DHAR, and AsA, DHA was observed in the current study. The ratio of AsA/DHA, GSH, GSSG, and the ratio of GSH/GSSG and proline content in tomato seedlings was observed to be affected by salinity, implying that tomato seedlings attempted to maintain redox regulation under salt stress. It was also confirmed at the transcriptomic level, as the relative transcript levels of antioxidant enzymes showed a similar trend with increasing salt stress duration. A similar gene expression pattern was previously observed in tomato under high temperature stress [
67,
68].
In addition, salinity stress also caused a significant increase in polyamines metabolism as suggested by the increase in the activities of three key enzymes named DAO, PAO and ADC. Similarly, in previous reports, salinity stress enhanced the ADC and ODC activities in tomato roots, indicating that both enzymes are responsible for stress tolerance [
69]. Furthermore, salinity stress also induced a slight increase in PAO activity [
70]. It has been well known that polyamines play important role under stress conditions by modulating ROS homeostasis and regulating the antioxidative mechanism to suppress the ROS production under stress conditions [
71]. Therefore, in the present study, the significant increase in enzymes involved in polyamines synthesis could be linked with the signaling regulation to enhance the responsiveness of tomato seedlings against salt stress.
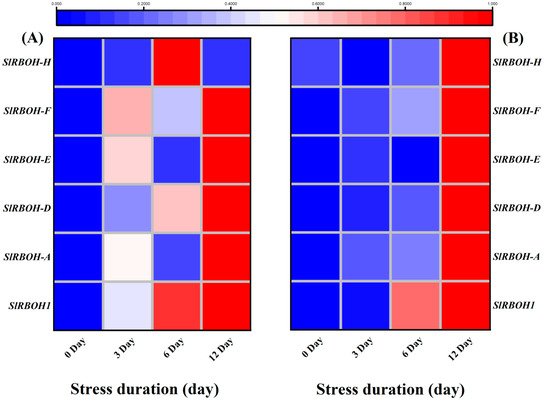
The plasma membrane-localized respiratory burst oxidase homolog (RBOH) proteins generate reactive oxygen species (ROS).
AtrbohD and
AtrbohF, the major NADPH oxidases responsible for ABA-induced ROS production in Arabidopsis, are involved in stomatal closure [
72].
OST1 interacts with
AtrbohD and
AtrbohF and phosphorylates Ser174 in
AtrbohF [
73]. To acquire further molecular insights into the salt tolerance mechanism of WT Ailsa Craig, we tested the expression of several TFs, ABA biosynthesis enzymes and signalling and defence-related proteins in WT seedlings. Combined with the accumulation of hydrogen superoxide radicals, it could be suggested that salt-induced over-production of H
2O
2 activated the RBOH signalling mechanism, which regulated the various adaptive mechanisms to counter the salt-induced osmotic stress in tomato seedlings. In previous studies with different abiotic stresses, including low temperature [
74], cold [
74] and salinity [
75], the acclimation to the stresses was found associated with the increased transcript levels of
RBOH1 [
76]. Furthermore, in Arabidopsis, the expressions of
AtrbohD and
AtrbohF were upregulated and Na
+/K
+ homeostasis in wild-type Arabidopsis seedlings that grow on MS medium containing NaCl [
77]. Previous studies with sugar beet revealed that
BvRBOHE,
BvRBOHF, and
BvRBOHH downregulates under salt stress. However, we found significant upregulation of both
SIRBOH-E,
SIRBOH-F after 12 days of salt stress and only
SIRBOH-H was found downregulated in roots after 12 days of salt stress. It suggests the significant role of RBOH transcription factors in tomato salt-stress acclimatization. Consistent with our results, a genome-wide identification of RBOH genes showed significant upregulation
RBOHA,
RBOHD,
RBOHF and
RBOHG under drought stress. Altogether, the significant upregulation of RBOH genes, particularly after 12 days of salt stress, strongly suggests their role in regulating the response of tomato seedlings to long-term salinity stress.
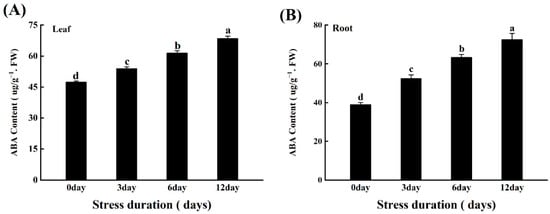
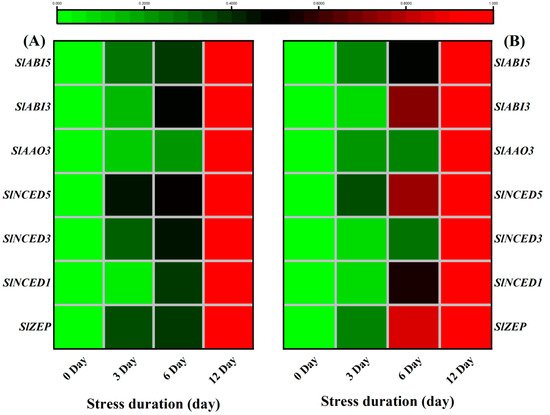
The phytohormone ABA plays a crucial role in regulating a range of plant physiological processes in response to various stresses [
78]. ABA functions as an essential secondary signalling molecule to activate a kinase cascade and mediate gene expression during salt stress [
79]. Osmotic stress, such as drought and high salinity, dramatically increases the ABA level, which induces the expression of many genes involved in stress responses [
80].
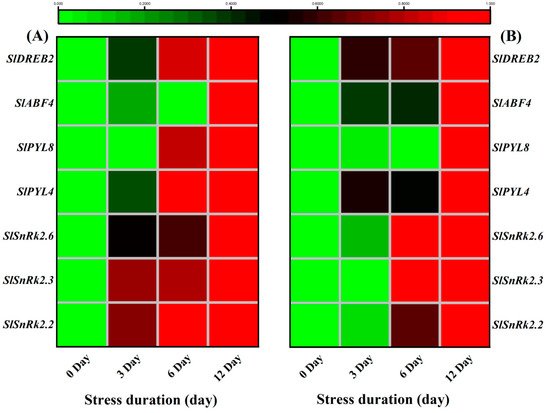
In addition to ABA-biosynthesis induction, salt stress also activated the downstream ABA signalling mechanism that controls ABA-regulated gene expression to enhance stress tolerance [
86]. Various gene families like SnRks [
87], DREBs [
88,
89], PYLs [
90] and ABFs [
91] are involved in ABA signalling that improves the tolerance of plants under stressful environments [
92]. Among SnRKs, Subfamily 2 of SNF1-related protein kinase (
SnRK2) is considered as a positive global regulator of abscisic acid signalling [
87]. Previously, it was noticed that
SnRK2.2,
SnRK2.6 and
SnRK2.3 activated the
AREB1/
ABF2,
AREB2/
ABF4, and
ABF3 as a response to osmotic stress at the vegetative stage [
93]. Therefore, the significant upregulation of the
ABF4,
PYL4 and
PYL8 in the present study could also be linked to the higher expression of SnrK genes to improve the ABA-regulated signalling against the salt stress. Consistently, a recent study showed the alterations in transcriptional levels of several salt stress-responsive genes like
SlPP2C37,
SlPYL4,
SlPYL8,
SlNAC022,
SlNAC042, and
SlSnRK2 family by
PpSnRK1α, signifying that SnRK1α is involved in the ABA signalling pathway to improve tomato salt tolerance [
94]. Functional analysis of the AREB/ABFs revealed that these proteins were positive regulators of the ABA-dependent signalling pathways under drought conditions [
95]. Similarly, physiological, biochemical and transcriptomic analyses showed that
SlDREB2 enhanced plant tolerance to salinity by improvement of K
+/Na
+ ratio and proline and polyamines biosynthesis [
96].