Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Cardiac & Cardiovascular Systems
Proprotein convertase subtilisin/kexin type 9 (PCSK9) is the last discovered member of the family of proprotein convertases (PCs), mainly synthetized in hepatic cells. This serine protease plays a pivotal role in the reduction of the number of low-density lipoprotein receptors (LDLRs) on the surface of hepatocytes, which leads to an increase in the level of cholesterol in the blood.
- Infections
- proprotein convertase subtilisin/kexin type 9
- SARS-CoV-2
- low-density lipoprotein receptors
1. Introduction
Proprotein convertases (PCs) are a family of nine serine proteases, which also includes proprotein convertase subtilisin/kexin type 9 (PCSK9). Each of those proteases plays a key role in post-translational modifications of propeptides leading to the formation of mature particles e.g., growth factors, enzymes, hormones, and transcriptional factors. Taking into consideration an ability for the activation of many substrates, to date, there seem to be a lot of physiological and pathophysiological processes that PCs take part in [1,2,3].
2. PCSK9 and Its Inhibitors
PCSK9, originally known as neural apoptosis-regulated convertase 1 (NARC-1), was discovered in 2003 by Seidah et al. [4]. It is synthetized in the endoplasmic reticulum (ER) of hepatic cells in the form of proenzyme, where after autocatalytic activation, it is transferred to plasma. The soluble PCSK9 binds to the epidermal growth factor homology domain A (EGF-A) of the low-density lipoprotein receptors (LDLRs) located mainly on the surface of hepatocytes, and makes them unable to attach low-density lipoprotein cholesterol (LDL-C) particles. In addition, it promotes the degradation of LDLRs by enhancing their endocytosis and blocking their recycling. Recent research shows that also intracellular PCSK9, before its exocytosis, can reduce the number of LDLRs (intracellular pathway) [2,5]. The targets for degradation by PCSK9 are also very-low-density lipoprotein receptors (VLDLRs) as particles from the close family to LDLRs [6]. The above-mentioned mechanisms lead to the impaired transport of cholesterol from the blood into cells and result in hypercholesterolemia. Furthermore, gain of function (GOF) mutations in PCSK9 genes are the cause of familial hypercholesterolemia with significantly higher levels of cholesterol in the blood [7], whereas loss-of-function mutations (LOF) are associated with hypocholesterolemia [8].
Among factors that affect the expression of PCSK9 we can single out: age, gender, diet, aerobic exercises, pregnancy, diurnal rhythm, diseases of thyroid gland, kidneys and liver, type 2 diabetes mellitus (DM2), obesity, drugs (e.g., statins) [10,11].
The clinical benefit from discoveries concerning PCSK9 influence on lipid metabolism, was the implementation to the therapy of hypercholesterolemia, medications that inhibit function of PCSK9–monoclonal antibodies against circulating PCSK9 (PCSK9 mAbs: alirocumab and evolocumab) and inclisiran—a small interfering RNA (siRNA) which prevents from the translation of messenger RNA (mRNA) of PCSK9 and in that way decreases the production of mature protein in liver [12,13,14].
Monoclonal antibodies against PCSK9 have the ability to lower plasma LDL cholesterol (LDL-C) by 60% from the baseline. They also decrease the level of apolipoprotein B (apoB) by 50% and triglycerides (TGs) by 15%, and have a slight positive effect on plasma high-density lipoprotein cholesterol (HDL-C) level and increase it by 5–10%. In contrast to statin therapy, they reduce (by even 25%) the concentration of lipoprotein(a) (Lp(a)), which is acknowledged as an independent cardiovascular risk factor [15,16]. The lipid-lowering potential of inclisiran is similar to PCSK9 mAbs, especially with regard to LDL-C and Lp(a) levels [17]. A potential advantage of therapy with inclisiran in the maintenance of compliance results from chemical structure and mechanism of action which ensure longer duration of lipid-lowering effects and, thus, less frequent administration of the drug in comparison with PCSK9 mAbs [18,19]. On the other hand, in spite of the high lipid-lowering potential of PCSK9 inhibitors, therapy with those drugs is still limited, mainly because of the high cost, strict reimbursement criteria and the COVID-19 pandemic, which results in a decline in number of patients that achieve therapeutic goals concerning LDL-C concentration [20,21].
Figure 1 shows the cellular mechanism of PCSK9 and its inhibitors on cholesterol metabolism [22].
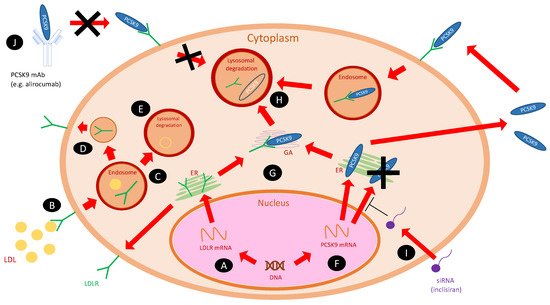
Figure 1. The cellular actions of PCSK9 and its inhibitors on cholesterol metabolism [22]. Abbreviations: ER, endoplasmatic reticulum; GA, Golgi apparatus; LDL, low-density lipoprotein; LDLR, low-density lipoprotein receptor; PCSK9, proprotein convertase subtilisin/kexin type 9; PCSK9 mAb, monoclonal antibody against PCSK9; siRNA, small interfering RNA (inclisiran). (A) LDLR gene is transcribed into mRNA, then translated within ER into mature protein and transferred on the cell surface. (B) LDL particles, after binding to the LDLRs on the cell surface, are internalized into an endosome. (C) As the endosome matures, LDL and LDLR decouple—LDLR (D) recycles, and LDL is catabolized in the lysosome (E). (F) PCSK9 gene is transcribed into mRNA and then translated within ER into the mature protein. It can bind to the LDLR intracellularly within Golgi apparatus (G) or be secreted to the extracellular space—both ways lead to lysosomal degradation of LDLR (H). (I) Inclisiran (siRNA) inhibits the translation of PCSK9 mRNA and prevents the formation of mature protein. (J) PCSK9 mAbs (e.g., alirocumab) bind to the soluble PCSK9 in the plasma and prevent from lysosomal degradation of LDLRs.
High efficacy in reducing the cardiovascular risk by PCSK9 mAbs observed in clinical trials (FOURIER, ODYSSEY Outcomes) seems not to be completely dependent on lipid-lowering effects [3]. The expression of PCSK9 in tissues not directly associated with cholesterol metabolism (e.g., endothelium, kidneys, pancreas, central nervous system), pleiotropic effects of other lipid-lowering drugs (e.g., statins) and multifunctional character of other proprotein convertases, were the reasons for proceeding studies on functions of PCSK9 that go beyond cholesterol metabolism [1,2,3,23,24]. In this article, we summarize current knowledge on functioning PCSK9 in different tissues and perspectives for its clinical use.
3. Bacterial Infections
Lipid molecules which form the structure of bacterial cell membranes, e.g., lipopolysaccharides (LPS) and lipoteichoic acid, play the leading role in initiating the host immune response against pathogens [191]. Studies show that the response intensity is significantly related to the concentration of those lipids, and limiting it prevents the occurrence of septic shock. The clearing of bacterial lipid structures from the bloodstream occurs with the participation of LDLRs [190,191,192].
Results from the experimental sepsis model showed that overexpression in PCSK9 was characterized by greater bacterial dissemination and consequent organ pathology, particularly in the liver and lungs [193]. Others have proven a significant reduction in proinflammatory cytokines concentration after LPS exposure in the group of patients with PCSK9 LOF mutations [194]. The above-mentioned data lead to a hypothesis that using PCSK9 inhibitors may improve the prognosis of patients with severe bacterial infections. Currently, this assumption is being verified in a randomized placebo-controlled double-blind study with alirocumab (NCT03634293). The study will assess the difference in survival after 28 days in patients with sepsis or septic shock, treated with PCSK9i or placebo in an intensive care unit (ICU). The results might introduce a novel group of drugs to the therapy of severe bacterial infections [195].
4. Viral Infections:
Flu-like symptoms and an increased number of upper respiratory tract infections have been reported as side effects in clinical trials with PCSK9 inhibitors [196,197]. So far, it has not been possible to explicitly confirm or contradict that PCSK9 is directly involved in the pathogenesis of viral diseases [198]. Considering the fact that the pathogenesis of viral infections involves many host factors, e.g., lipids and lipoproteins [199], the influence of PCSK9 inhibitors on the course of viral infections has captured the attention of many scientists all over the world.
4.1. Hepatitis C Virus
Hepatitis C virus (HCV) infection is a crucial cause of severe liver disease leading to chronic hepatitis, cirrhosis and hepatocellular carcinoma. The World Health Organization estimates that seventy-one million people have HCV infection worldwide and at least 400,000 people die of it every year [200].
HCV enters hepatocytes with the use of different proteins—one of them is LDLR [201]. Syed GH et al. observed that hepatic cells infected by HCV contain more LDLRs than those from the control group [202]. Some studies suggested that LDLR also plays a key role for the post-entry viral processes such as replication [203]. Furthermore, the report from an in vitro study revealed that LDLR degradation by PCSK9 with the participation of hepatic CD81 particle is one of the main ways in which HCV invades cells [204,205].
These results raised the concern that PCSK9 inhibitors might increase CD81 levels, and thereby susceptibility to HCV infection. In 2016, Ramanathan A. et al., in their in vitro and in vivo studies, did not confirm that treatment with alirocumab increased the risk of HCV infection or CD81 levels [206]. Moreover, analyses of the results of ten clinical trials, which compared the action of alirocumab with placebo or ezetimibe, showed no elevations in predisposition to HCV infection in the groups of participants treated with PCSK9i. [206] Additionally, the ODYSSEY and FOURIER clinical trials did not reveal statistically significant increases in the incidence of hepatic disorders [207,208].
4.2. Dengue Virus
Dengue fever is one of major global health problems, with 390 million individuals infected annually, and with a rate of mortality that may reach up to 20% [209,210]. Dengue virus (DENV) requires cholesterol in the development cycle: viral entry by membrane, fusion, and replication [211]. In vitro and animal models with statins revealed the reduction in DENV infections [212,213]. Unfortunately, lovastatin used in a clinical trial did not repeat this effectiveness in human [214]. New hopes appeared with the efficacy of lipid-lowering drugs from the group of PCSK9 inhibitors. Gan ES et al. reported that elevated PCSK9 levels are associated with higher viremia and an increased risk of a more severe course of dengue [215]. Clinical trials with PCSK9 inhibitors are required to test their potential effectiveness in a novel indication.
4.3. SARS-CoV-2
An interstitial pneumonia caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was first reported in Wuhan, Hubei, China, in December 2019. As of 14 January 2022, over 318 million SARS-CoV-2 infections have been reported. So far, 5.5 million deaths have been associated with coronavirus disease 2019 (COVID-19). Cardiovascular diseases, arterial hypertension, hyperlipidemia, diabetes, kidney and chronic lung diseases, cancer and obesity have all been associated with severe course of the disease and increased mortality rate [216,217].
SARS-CoV-2 enters host cells using angiotensin converting enzyme 2 (ACE2) receptors and with the participation of cholesterol in trafficking ACE2 to the viral entry site [218]. There are some hypotheses suggesting that PCSK9 inhibitors, as the lipid-lowering drugs, could affect various elements in the pathophysiology of SARS-CoV-2 infection [218].
COVID-19 is associated with increased incidence of coagulation abnormalities and higher risk of venous thromboembolic disease [219]. An analysis of clinical trials (FOURIER and ODYSSEY OUTCOMES) reported lower rates of venous thromboembolism in patients who were treated with PCSK9 inhibitors compared with placebo [207,208].
Systemic endothelial inflammation is considered as one of the main causes of severe course of SARS-CoV-2 infection [220]. The positive correlation between LDL plasma concentration and endothelial dysfunction is widely known [221]. Some studies have confirmed that PCSK9 inhibitors improves the functioning of the endothelium in patients with inflammatory diseases [222,223]. In the ODYSSEY FH I and FH study, PCSK9 inhibitors decreased the level of the atherothrombogenic Lp(a) by approximately 30% [224]. The observation by Huang W et al. showed a negative correlation between PCSK9 expression and the risk of infection with SARS-CoV2 [225]. Further studies are required to unequivocally assess the potentially beneficial effects of PCSK9 inhibitors in the therapy for COVID-19.
5. Parasites
Parasites the organisms that need cholesterol to develop properly. As they lack the ability to synthesize it de novo, they use cholesterol from the host [226].
Arama C et al. examined the influence of GOF mutations in PCSK9 on the course of malaria. The study revealed that in a group of 752 Malians children, those who were GOF mutation carriers were prone to a more severe course of disease [227]. In the research of Fedoryak O et al., LOF mutations in PCSK9 were associated with reduced mortality in the course of malaria [228]. According to their results, a hypothesis that PCSK9 inhibitors could be useful for the therapy and prevention of malaria was formed. To date, there is no scientific evidence confirming the association between the therapy with PCSK9 inhibitors and the course of malaria. Further studies are required.
This entry is offline, you can click here to edit this entry!
