Clinical trials for Alzheimer’s disease (AD) face multiple challenges, such as the high screen failure rate and the even allocation of heterogeneous participants. Artificial intelligence (AI), which has become a potent tool of modern science with the expansion in the volume, variety, and velocity of biological data, offers promising potential to address these issues in AD clinical trials. In this review, we introduce the current status of AD clinical trials and the topic of machine learning. Then, a comprehensive review is focused on the potential applications of AI in the steps of AD clinical trials, including the prediction of protein and MRI AD biomarkers in the prescreening process during eligibility assessment and the likelihood stratification of AD subjects into rapid and slow progressors in randomization. Finally, this review provides challenges, developments, and the future outlook on the integration of AI into AD clinical trials.
1. Introduction
Recent advances in understanding the neurobiology of Alzheimer’s Disease (AD) revealed that the initiation of disease processes leading to symptomatic and functional neurodegeneration precedes the onset of dementia by 15–20 years [
1,
2]. The amyloid cascade hypothesis explains that beta-amyloid (Aβ) triggers the following procession, such as the development of neurofibrillary tangles (NFTs), cortical atrophy, cognitive impairments, and loss of activities of daily living [
3,
4,
5]. These AD biomarkers appear in the predementia stage, including normal cognition (NC) and mild cognitive impairment (MCI). Thus, previous clinical trials have focused on the development of Aβ targeting diagnostic and therapeutic methods. There are also growing clinical trials targeting tau and NFTs, as tau pathology is more closely correlated with cognitive decline than Aβ [
6].
Despite the stagnancy in AD clinical trials for the past 18 years ever since memantine was launched in 2003, a recent clinical trial of Biogen’s aducanumab has demonstrated a statistically significant reduction in Aβ plaques [
7,
8]. The US Food and Drug Administration has approved aducanumab for AD treatment using the Accelerated Approval pathway, which is expected to serve as an impetus for global AD clinical trial efforts. AD clinical trials involve two notable steps: eligibility assessment and randomization.
Furthermore, given the heterogeneous rates of AD progression, it is important to allocate subjects evenly into intervention and control groups based on their AD trajectories for a reliable observation of the treatment [10,11].
Artificial Intelligence (AI) refers to “the ability of a digital machine or computer to accomplish tasks that traditionally required human intelligence.” [
12] A convergence of advanced AI algorithms, data proliferation, tremendous increases in computing power, and memory storage has propelled AI from hype to reality. ML algorithms could enhance the ability to detect hidden structures or underlying patterns of the data to improve the performance over time and learn how to make a prediction rather than explicit instruction.
2. Eligibility Assessment
Screening is an important process in AD clinical trials to ascertain that selected participants are only those with AD pathology. Clinical diagnosis of AD follows the 1984 NINCDS-ADRDA Work Group criteria [
17] or the 2011 NIA-AA guidelines [
18]. Recent studies have shown that 15–25% of clinically diagnosed AD patients showed incompatible amyloid positron emission tomography (PET) or cerebrospinal fluid (CSF) findings [
19,
20]. Additionally, the increasing tendency of AD clinical trials to target preclinical stages, where cognition and functionality are normal, has underscored the importance of biomarker-guided screening. However, AD clinical trials have a high screen failure rate and corresponding low recruitment rate, as only one-third of asymptomatic older adults are Aβ+ [
21]. Therefore, prescreening algorithms using AI could help reduce screen failure rate by classifying the recruited population into high and low likelihood groups, with the former undergoing screening procedures for validation and the latter being excluded from the clinical trials (
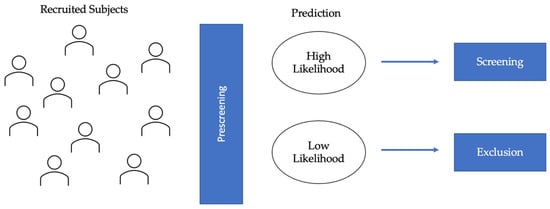
Figure 1). This consideration applies to clinical trials for both disease-modifying therapies (DMTs) and symptomatic treatments.
Figure 1. Diagram of eligibility assessment in AD clinical trials. AI-applications in eligibility assessment would prescreen the recruited subjects to identify the high likelihood and low likelihood groups. AI algorithms would be used to classify individuals based on predicted protein (Aβ and tau) biomarkers and/or MRI biomarkers. The high likelihood group would be selected for further screening, and the low likelihood group would be excluded, thereby leading to lower screen failure.
2.1. Protein Biomarkers for AD
Although causal mechanisms remain unclear, Aβ and tau proteinopathies are defining features of AD as a unique disease [
22]. AI prescreening algorithms can reduce challenges of PET and CSF, such as high costs and participants’ fear of radiation exposure, by selecting a subset of individuals who are likely to be Aβ or tau positive. Therefore, in AI research for AD clinical trials, many aimed to predict amyloidosis in subjects with MCI [
23,
24,
25], while others focused on preclinical stages before neurodegeneration is too substantial [
26,
27].
2.2. MRI Biomarkers
Within the A/T/N framework, neurodegeneration reflects downstream effects of molecular AD pathology, closely correlating with cognitive and functional decline [
56,
57]. Among many neuroimaging modalities, structural MRI (sMRI) is widely used as a surrogate marker for neurodegeneration due to its relative availability, low costs, and good diagnostic accuracy [
58,
59,
60,
61]. Therefore, many have used AI to capture the spatial patterns of atrophy in MRI data to enhance the linkage between neurodegeneration and AD-related changes. MRI can reveal the anatomical differences between AD and NC to classify subjects into different stages of AD [
62,
63,
64]. It can also detect MCI subjects who will convert to AD based on the temporal link between MRI abnormalities and the onset of cognitive impairment [
65,
66,
67]. MCI subjects who convert to AD during the duration of the study are often categorized as pMCI, while those who remain in MCI or revert to NC are categorized as sMCI.
3. Randomization
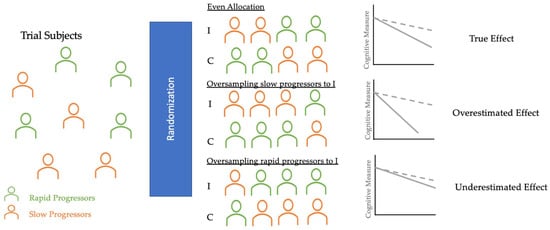
Primary outcomes of AD clinical trials are often the absence of clinical progression (measured by scales, such as the Clinical Dementia Rating (CDR)) or cognitive deterioration (measured by NP test scores). However, many longitudinal studies have identified fast and slow AD progressors characterized by heterogeneous rates of cognitive and functional decline [
97,
98,
99,
100]. Randomization in clinical trials does not always allocate an equal proportion of rapid and slow progressors into control and intervention groups [
101,
102]. As
Figure 2 shows, if rapid progressors are mainly selected for the intervention group and slow progressors for the control group, the reported treatment effect would seem as though the treatment had no significant impact, even if the treatment was, in fact, clinically efficacious. On the other hand, if slow progressors were mainly selected for the intervention and rapid progressors for the control group, the reported treatment would have overestimated the clinical efficacy. Therefore, an even allocation of rapid and slow decliners into intervention and control groups is desirable to reduce bias in treatment assignment and avoid the two aforementioned extreme scenarios that could explain the failures [
103] and successes [
104] of AD clinical trials in the last two decades.
Figure 2. Diagram of randomization in AD clinical trials. These three potential scenarios of randomization illustrate how an uneven allocation of rapid or slow progressors (oversampling or undersampling) into I and C can obscure the true treatment effect. Thus, AI applications in randomization can classify trial subjects into these clusters and help trials achieve even allocation. I, Intervention group (dotted line); C, Control group (solid line).
4. Challenges and Future Directions
AI systems with large-scale data have facilitated the development of disease prediction that can potentially reduce the screen failure rate of clinical trials [
39]. Furthermore, identifying suitable participants in trial recruitment contributes to reducing associated expenses and accelerates drug developments [
117]. However, it is important to acknowledge several challenges for its applications in clinical trials.
Advanced AI models derived from high-quality databases often showed good predictive performance; additional information from explainable and transparent AI technology might further the understanding of biomedical data and improve their applications in clinical trials. A common form of a visible machine learning algorithm, such as a graphical neural network might provide structural connections between different medical entities (e.g., diseases, drugs, and proteins). For example, GNNexplainer identifies a small set of important variables and genetic pathways that contribute to human disease [
118]. Identification of disease mechanisms through the multiscale interactome has facilitated efficacious and safe therapeutic development. In addition, earlier access to the drug candidates could help improve the time and expenditure of prescreening process in clinical trials. Thus, developing an explainable and transparent AI system would substantially benefit both the speed and efficiency of clinical trials and drug discovery.
6. Conclusions
Clinical trials for AD face challenges of high screen failure and even allocation of the heterogeneous subject population. Many recent works have investigated the potential applications of AI to address these challenges in clinical trials, particularly in the steps of eligibility assessment and randomization. The prediction of protein and MRI AD biomarkers in the prescreening process could drastically reduce the high screen failure rate. Additionally, the AI-based stratification of the AD subject population into rapid and slow progressors can guide the even allocation of the heterogeneous AD population into intervention and control groups during randomization. AI algorithms have not been integrated into AD clinical trials due to the lack of explainability and poor external and internal validations. However, integrating biological knowledge to develop the multiscale interactome and rigorous external validations for generalizability and reproducibility could result in novel diagnostic biomarkers and precision medicine.
This entry is adapted from the peer-reviewed paper 10.3390/life12020275