The present review deals with bioactive glasses, a class of biomaterials renowned for their osteoinductive and osteoconductive capabilities, and thus widely used in tissue engineer-ing, i.e. for the repair and replacement of damaged or missing bone. In particular, the paper deals with applications in periodontal regeneration, with a special focus on in vitro, in vivo and clinical studies. The research was based on Preferred Reporting Articles for Systematic Reviews and Meta-Analysis (PRISMA). The study reviewed eligible publications, identified on the basis of inclusion/exclusion criteria, over a ranged time of fifteen years (from 01 January 2006 to 31 March 2021). While there are many papers dealing with in vitro tests, only a few have reported in vivo (in animal) research, or even clinical trials. Anyways, BGs seem to be an adequate choice as grafts in periodontal regeneration, in particular using the recently described minimally invasive surgical techniques that were studied to treat the perodontal defects with the graft alone and without membranes that require the periosteal fenestration and a coronlally advanced flap, as a rule.
1. Introduction
Bioactive glasses (BGs) were discovered by Larry L. Hench in 1969; the original composition, named Bioglass
® 45S5 (45S5 from here on) [
1,
2] displayed outstanding properties such as bone regeneration capabilities and antibacterial activity. In fact, such BG is an amorphous and biocompatible silica oxide-based inorganic material able to induce surface property responses resulting in the formation of a bond between the bone and the glass itself. From 45S5, a family of BGs was developed; such glasses are usually composed by oxides of Si, Ca, P, and Na. The release of these ions into the tissues could induce specific cell responses and explain their biologic properties [
3]. In recent years, such compositions have been further modified to incorporate also the so-called therapeutic ions. Thus, strontium, magnesium, copper, silver, zinc, lithium have been successfully included in BG formulations, and the resulting BGs displayed favorable effects in terms of overall biological response [
4,
5,
6,
7,
8,
9,
10]. BGs could regulate gene expression, protein synthesis, and cell-mediated mineralization [
11]. Moreover, the incorporation of metallic ions into BGs induces angiogenesis [
12].
Even if silica oxide-based glasses are the most used, other systems were also developed, such as phosphate-based BGs. These glasses are mainly based on P
2O
5, Na
2O, and CaO and modifying oxides, for example CuO [
13], ZnO [
14], Ag
2O [
15], Fe
2O
3, TiO
2 [
16], and SrO [
17], which improve their stability [
18]. They have degradation times ranging from hours to years depending on the composition and can be used as a scaffold for tissue regeneration [
19].
In general, the surface of BGs, when soaked in physiological fluids, undergoes a complex ion exchange mechanism with the medium, inducing the formation of precipitates and subsequently hydroxyapatite deposition. This mechanism could explain the effectiveness of these BGs to bind to bones and the wide number of studies on BGs as supporting materials for bone tissue engineering and tooth remineralization [
20]. Moreover, this superficial layer plays an important role in favoring cells migration and adhesion. Thus, by the same mechanism of ion exchange, bioactive glasses have been shown to regulate gene expression and promote cells differentiation, two important steps in tissue regeneration and repair [
21].
BGs can be produced by melt-quenching or sol-gel routes [
22]. In the first method, the oxides (or their precursor such as nitrates or carbonates) are melted together and rapidly quenched to obtain a frit, which is subsequently ground and sieved. The sol-gel route requires a specific chemical approach with precursor polymerization at room temperature to form the glass network [
23]. Even if the melt-quenching route is the most employed, sol-gel offers some advantages, i.e., the synthesis temperature is relatively low, the composition ratio can be easily modified, the standardization of the product’s particle size can be organized to obtain pure samples with high uniformity, and constituents are allowed to be doped [
24].
BGs could be prepared with a different degree of porosity, increasing the surface area, providing different patterns of support for cell adhesion, cell migration, and modifying cellular response and growth [
25]. Indeed, the porosity could act as a guide for angiogenesis and trophic supply [
26].
BGs show a great versatility of composition and, consequently, of use. Up to date, BGs have been produced in bulk form, particles or nanoparticles, granules, scaffolds, and coatings (e.g., [
27,
28,
29,
30,
31,
32,
33]). As far as the coatings are concerned, BG can be deposited on metallic substrates, such as prostheses or dental implants, to improve the biological behavior of the system; in this case the analysis of potential residual stresses due to the mismatch of coefficients of thermal expansion between the glass and the substrate should be included [
34,
35].
Moreover, BGs can be combined with other materials to obtain bioactive composites [
36], or their porous structure could be loaded with bioactive molecules for a local release of active principles [
37]. However, not all substances can be favorably mixed with BGs, e.g., polylactic acid undergoes a biodegradation producing an acid environment that neutralizes the alkaline environment generated by BGs, favoring bacteria proliferation [
26,
38]. On the contrary, a BG containing Zn and Mg was incorporated into alginate networks to improve mechanical, antibacterial, and biologic properties in dentistry [
39]. The use of chitosan in dentistry [
40,
41,
42] represents a novel choice. Chitosan is neutral after degradation, so the alkaline antibacterial environment proper of BG can carry out its function. This composite material has the potential to induce bone regeneration and could be significant for promoting the proliferation and metabolism of human periodontal ligament cells and the metabolism of human bone marrow stromal cells [
38].
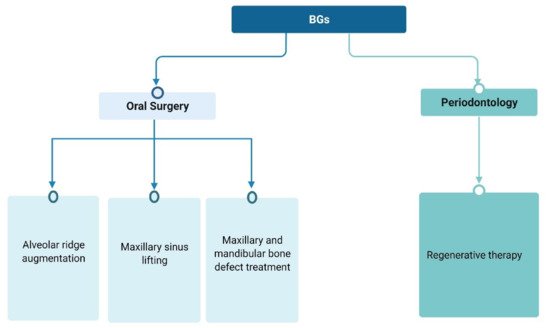
In tissue engineering, a major focus of attention is the recognition and activation of adult stem cells. Periodontal tissue harbors a great amount of different cell types. Inside the periodontal tissue, mesenchymal cells and adult stem cells have been identified and have been demonstrated to be able to differentiate in periodontal ligament specific fibroblasts [
43,
44,
45]. The main role of BGs in dental and periodontal restoration and regeneration is the proper stimulation of those cells, to produce new tissues and to favor periodontal attachment. In fact, an ideal material for periodontal regeneration should be able to elicit and promote cell proliferation and differentiation and should have adequate mechanical properties, resembling the target tissue. Additionally, the material should be suitable for the different clinical features of the possible periodontal defects (as illustrated in
Figure 1).
Figure 1. BGs use as regenerative surgical treatment in dentistry.
Moreover, BGs must show antibacterial or bacteriostatic properties and interesting interplays with the immune system.
BGs seem to hinder bacterial growth and development, and this task can be improved by loading them with antibiotics or doping with bactericidal ions (such as silver) to avoid the emergence of resistant strains [
12]. Increasing evidence reveals the mechanisms and cellular pathways of interactions between BGs and the immune system. In particular, the influence of BGs on immune cells (e.g., macrophages, monocytes, DCs) has been demonstrated, as a key factor to regulate the immune response to these biomaterials. The interaction between BGs and the immune system (especially with macrophages and monocytes) appears to be bi-univocal and dynamic. The secretion of specific cytokines by immune cells seems to be significantly affected by the degradation kinetics and degradation products (mostly ions) of BGs [
46]. In this context, it should be pointed out that immune cells can conversely influence the degradation process of BGs, which consequently affects the structure, morphology, mechanical properties, and ion release behavior of BGs. However, to develop feasible, effective, and advanced BG-based grafts or also biomedical devices with immunomodulatory capability for tissue regeneration, several major challenges remain and have to be addressed in future studies [
47].
Periodontal diseases are oral infections characterized by gingival inflammation, clinical attachment loss, and alveolar bone resorption [
48]. Oral microbiota represents one of the most effective risk factors for periodontitis and for periodontal regenerative therapy failure [
49,
50]. The current trend in periodontology is to preserve as much as possible the periodontal attachment and to regenerate the lost parts. Therefore, tissue engineering is a topical subject and an emerging field based on the combination of advanced surgical techniques, stem cells, growth factors, angiogenesis, and biomaterials.
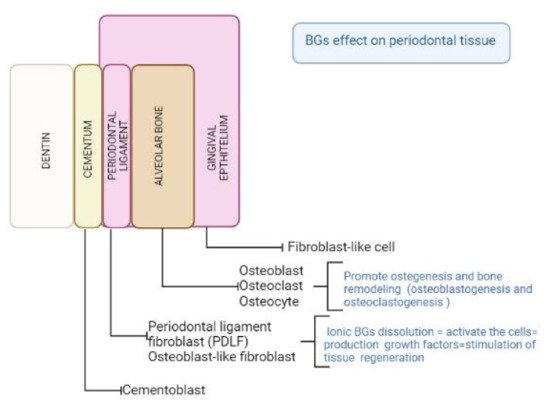
Since BGs show intriguing properties considering their biocompatibility, cell response, adaptability to clinical features and antibacterial properties, they have been incorporated as filler in scaffolds for tooth and periodontal regeneration [
24] (
Figure 2).
Figure 2. Schematic illustration of the BGs effect on periodontal cells.
2. In Vitro Tests
In recent years, in vitro studies have been mainly focused on: (1) the analysis of materials for the treatment of bone defects, (2) the addition of chemical elements to the bioactive glass already in use to enhance its osteo-regenerative effect, (3) the effect of new BG production techniques that could also influence its osteoinductive capacity, (4) the creation of new composites/membranes/scaffolds for guided tissue regeneration/bone regeneration.
We know that the periodontal area is histologically represented by four connective tissues, of which two are mineralized (cement and alveolar bone) and the other two are fibrous (lamina propria of the gingiva and periodontal ligament) [
103]. In particular, the periodontal ligament is a peculiar connective tissue that contains at least three distinct populations of cells: fibroblasts, osteoblasts, or cementoblasts.
Regarding the cell types used in the cited papers, human and mouse cells were used, both of primary and immortalized origin.
The cells were placed in direct contact with the materials or with eluates/extracts of the material, to assess their cytocompatibility and/or cell attachment and/or cell proliferation and/or osteoinductive capacity.
Human periodontal ligament fibroblasts (hPDLF), taken from the patients for the study, were the most commonly used cells in in vitro studies for the evaluation of bioactive glass in periodontal bone regeneration.
Fibroblasts of gingival origin, such as human gingival fibroblasts (hGF), have also been used in some in vitro studies.
In general, in our bibliographic research, cellular fibroblasts (deriving from the periodontal ligament or from the gingiva) were the most used to verify aspects of cytocompatibility and bone regeneration using bioactive glasses.
Human primary osteoblastic cells (POBs) derived from bone were also used. Such cells are widely utilized because they are easy to buy and do not require the approval of an ethics committee. POBs are isolated from the femoral trabecular bone tissue from the knee or hip joint region.
Other purchasable bone-derived cells are: (1) the human osteoblast (hOB) cell line, isolated from fetal or adult human bone, recognized as a model system for skeletal system studies, (2) immortalized cell lines such as the human osteosarcoma cell line (MG63), and (3) human primary osteogenic sarcoma (Saos-2 cells); the latter are widely used as osteoblastic cell models; (4) human dental pulp stem cells (hDPSC) isolated from human third molar teeth, which were also used to evaluate cytocompatibility and osteogenic differentiation.
In many studies cells of non-human origin, such as mouse or rat cells, were frequently used; in one paper we found that hamster cells were used.
The murine osteoblastic cell line (MC3T3-E1) was reported in various studies, being an osteoprogenetic line. Such line is widely used to evaluate (i) how the materials (e.g., BGs) act on the differentiation from osteoblasts into mature osteoblast phenotypes and (ii) osteogenic gene expression.
As cells included in the standard cytotoxicity tests (ISO 10933-5), the mouse fibroblasts (L-929) are immortalized cells derived from subcutaneous connective tissue, and only one paper between the one analyzed used them [
69] to evaluate the cytotoxicity of materials’ extracts according to regulations.
Mouse dental papilla (MDPC-23) cells, a spontaneously immortalized cell line, are derived from fetal mouse molar papillae and are widely used in many in vitro studies for dentistry; such cells are referred as odontoblastic cell lines.
The in vitro studies found on murine cells were more specific; cell lines in the periodontal setting, cells of the parodontal ligament (rat periodontal ligament stem cells (pDLSCs)), and Wistar rats gingival fibroblasts (rGF) were used. Alternatively, dental but non-periodontal specific cells were used, such as rat pheochromocytoma PC-12 cells dental pulp cell line (KN-3), rats cementoblast (rC), rat primary osteoblastic (rPO) cells, rat osteoblasts calvaria (rOC).
Chinese hamster ovarian cells (CHO cell Line) were used for assessment of the cytotoxicity effects by Esfahanizadeh et al. [
63].
With regard to the secondary outcomes, different authors found that, when human cells were used, BGs induced and/or increased cell viability and proliferation, and stimulated mineralized tissue formation [
37,
53,
55,
66,
67,
72,
75], especially when BG nanoparticles were employed [
55,
66]. When fibroblast/osteocyte cell lines were utilized, cell viability, cell proliferation, cell differentiation were appreciated [
60,
64,
70,
71], in particular with mesoporous BG nanospheres/nanoparticles [
54,
68]. Some studies detected specific cell factors involved in the osteoinduction response [
37,
56,
59], while BGs in membranes promoted healing [
64,
65,
66,
68,
69]. Finally, antibacterial activity of BGs was demonstrated [
52].
In general, the studies reported in this review to evaluate cytocompatibility and regenerative osteoinduction in vitro show heterogeneity in the cell types used and, more importantly, employ mostly non-periodontal and non-dental cells. The use of such a wide range of cells, of culture systems based on primary cultures or on immortalized lines, and different study designs makes it difficult to blend the results regarding the studies considered. Almost always, these studies performed tests aimed to analyze cell viability, cell proliferation, cell differentiation, and enhanced mineralized tissue formation that correspond to the selected secondary outcomes mentioned above. Nanostructured BGs and/or composites with BGs, in particular doped with boron, strontium, or niobium, seemed to guarantee better results considering the secondary outcomes. Zn or Ag could induce better in-vitro antibiofilm activity, with the important limits described above of their clinical significance.
3. In Vivo Tests
Different animal models were used in the studies: dogs, rabbits, mice, rats, with different follow-ups.
Of seven articles, three used the dog as an animal model, with a low number of animals.
Felipe et al. [
77] implanted BG particles in six dogs, Lee et al. [
78] utilized calcium phosphate glass in five dogs, and Carvalho et al. [
76] investigated Perioglas
® and PRP in nine mixed-breed dogs.
Thus, different models of periodontal defects were analyzed in the three articles using the dog as an animal model, with different prognosis, different healing predictivity (e.g., 3-wall defects vs. critical size defects) and thus requiring different surgical strategies to be regenerated. Moreover, different healing times (60 days, 90 days) were used. On the contrary, the histological and histomorphometric analysis was the same in all the papers.
Another animal model used in the in vivo studies to evaluate BGs in surgical therapy of the periodontal defect was the rabbit [
59].
With regard to smaller animal models, the mouse/rat model was used. It is certainly a cheaper animal model, which allows an increase in the number of animals. These animal models compel to use a quite different dental context than the Primates’ one, or just an anatomical context different from the oral one, adding—to the difficult comparisons with the human periodontal disease model—the disturbing variable of the different anatomical site, where certainly there is no periodontal ligament. Therefore, in the few studies found the materials are implanted in different anatomical areas, calvaria in the mouse and calvaria, dorsal muscles, and mandible in the rat.
Granel et al. [
71] showed that more than 30% of defect repair occurred after 90 days using BG-PCL scaffolds than the control sites left empty, with homogeneous mineralization and support bone remodeling.
Shah et al. [
68] did not evaluate bone regeneration because no bone defect was created, but only a subcutaneous pocket; a membrane with BG was inserted in Wistar rats. The evaluation after one month only verified the good histocompatibility of the implanted material.
Fifteen wistar rats were used by Zhang et al. [
79] as animal model for the in vivo study of a BG scaffold and a scaffold made of BG containing also strontium. Bilateral defects in the mandible were created and then filled with the biomaterial divided in three groups: non-treated control, BG scaffold, and strontium-BG scaffold. After 28 days from implantation the periodontal fenestration defects treated with strontium containing BG scaffolds showed greater new bone formation (46.67%) when compared to BG scaffolds (39.33%) and control unfilled samples (17.50%). The study showed that the use of strontium doped BG scaffolds decreased osteoclastogenesis, plus increased alveolar bone regeneration.
In these few articles found, the variability in the surgical procedure, in the follow-up times and in the different use of the BGs does not allow to properly compare the effect of BGs in the dog animal model. However, some considerations are possible. BGs have been demonstrated to achieve clearly better results than controls, especially with regard to bone regeneration. This can be ascribed also to the possibility of varying the composition of BGs and/or of producing composites. Strontium-doped BGs appear to guarantee better regenerative outcomes [
79], as well as association with PRP or growth factors [
59,
76]. In particular, zinc seems to allow the control of the dissolution and replacement times of BGs in biological tissues; additionally, the release of zinc ions accelerates bone formation [
104,
105]. Another consideration concerns the use of membranes for GTR. Within the limitations of the studies available, it seems that the use of membranes in GTR both with particulate BGs and scaffolds was not of particular importance. This aspect significantly differentiates BGs from hydroxyapatite-based materials. Moreover, to envelope [
59] or not [
104] BGs by membranes did not influence the inflammatory outcome, that seemed to be feeble.
4. Clinical Studies
There are several topics regarding periodontal regenerative therapy [
106]. However, considering exclusively the periodontal defect to be treated, the main issues are the surgical techniques, the materials used to treat the periodontal defects and the results over time [
107,
108,
109,
110,
111,
112]. The surgical strategy depends on the periodontal defect that has to be regenerated. The material used has to favor at least the regeneration of the periodontal ligament and of the alveolar bone. The stabilization of the results over time is considered in relation to the follow-up of the study design. This last parameter, which from a clinical point of view is the more valid the more extensive, has its rationale in relation to the chosen regenerative system.
Despite a recently published definition regarding short- (6–12 months), medium- (13–59 months), and long-term (>5 years) periodontal surgery [
112], undoubtedly most of the studies on the outcomes of periodontal regeneration procedures are concluded at 6 months or 1 year. The primary aims of the periodontal regenerative surgery consist in providing the clot mechanical stabilization in the regenerative space, allowing angiogenesis and avoiding the infection. The periodontal intrabony defects (which are considered in this review) could be self-maintaining space defects [
113], i.e., intraosseous defects provided of a bone morphology fully defining the mechanical stabilization of the clot, or non-self-maintaining space defects. In last clinical cases, the degenerated tissue content, proper of the periodontal defect (periodontal pocket), is not removed (the provided surgical therapy is limited to the thorough removing of the only subgingival microbiota ecosystems); in alternative, if the pocket tissue is removed, a complementary system has to be provided to maintain the regenerative space; an exoskeleton or an endoskeleton system are possible choices [
109]. The advantages of titanium reinforced PTFE membranes may therefore be related to space provision and to blood clot stabilization effects (exoskeleton system). Resorbable membranes do not have the same self-maintaining space characteristic and may be used alone only in contained defects. Non-contained defects treated with resorbable membranes may therefore benefit from the combined use of a grafting material acting as a scaffold (endoskeleton system) [
86,
109]. Mostly, the filling materials should not be grafted alone. The covering with membranes is needed, in particular if the graft consists in an alloplastic HA (e.g., demineralized bovine bone mineral—DBBM) [
114].
Alternatively, grafts have to be combined with other compounds increasing their osteogenetic properties and stabilizing the graft shape against mechanical stresses (e.g., amelogenins, platelet-rich plasma, recombinant human platelet derived growth factor-BB, peptide P-15, etc.) [
109,
115]. Clinical outcomes of the use of bioactive agents when applied in addition to OFD, either alone or in association with grafts and/or barrier membranes, were evaluated. The studies concluded that there was evidence to support the use of amelogenins, either alone or in combination with grafts, to treat intraosseous defects effectively, and the additional use of a graft seemed to enhance the clinical outcome of amelogenins.
Deep intrabony defects with high self-maintaining space properties show the same regenerative clinical outcome by the only minimally invasive surgical techniques, as minimally invasive surgical technique [
116] or single flap approach [
117] without grafting or membrane covering (exoskeleton or an endoskeleton systems). Strictly speaking, the open flap debridement (OFD) surgical technique consists of quite a different surgical approach. It is considered a periodontal surgical procedure in which the supporting alveolar bone and root surfaces of teeth are exposed by incising the gingiva to provide increased access for scaling and root planing (the provided surgical therapy is limited to the thorough removing of the only subgingival microbiota ecosystems). While the efficacy of this treatment is debated, it is performed ancillary to any osseous resective or regenerative periodontal procedures. So, OFD is not a surgical approach specifically suitable for resective or regenerative procedures. Besides, a large flap, extended to the neighboring teeth and including also an eventual periosteal incision and/or vertical-releasing incisions, will be chosen in the presence of a severe and deep defect, involving three or four sides of the root, requiring ample visibility for instrumentation or/and the use of either endoskeletons and membranes (resorbable exoskeletons) or non-resorbable exoskeletons [
109]. However, a wide series of specific surgical techniques are commonly identified as OFD.
Most of the clinical trials considered in this review, covering the last 15 years, are randomized controlled trials. Mostly, the papers considered patients suffering from chronic periodontitis and all patients were systemically healthy. The OFD was often the chosen surgical technique, and it was carried out with the removal of the tissue from the periodontal pocket. Considering clinically correct the chosen surgical technique through force of circumstance, all the studies considered highlighted a clinical advantage using BGs graft than the alone OFD along time, varying from 6 to 9 months [
80,
87,
89,
90]. In all the cases, the BG graft (endoskeleton) was not provided with a covering membrane and in one case it consisted of a BG reinforced HA with α and β forms of tricalcium-phosphate [
81]. So, if the intraosseous defects were provided of self-maintaining space properties, the short-term follow-up was appropriate to demonstrate the healing acceleration effect (which is what can be detected with these study designs). Moreover, BG may not show the same disadvantage as alloplastic HA grafts, which would need to be combined or covered with membranes. On the other hand, if the OFD had been implemented with the removal of the pocket tissue in non-self-maintaining space defects, the favorable results obtained with the graft would derive most of all from a methodological bias.
The use of BG has been evaluated with different study designs, comparing or combining BG with compounds capable of generating periodontal regeneration (enamel matrix protein derivative—EMD, platelet pellet—PP, platelet-rich plasma—PRP) but without the mechanical properties to maintain the space of the periodontal defects. The studies showed a follow-up from 6 months to 5 years [
81,
82,
83,
84,
86,
95,
96,
97]. Mostly, the studies highlighted for both the grafted materials (BG vs. EMD/PP/PRP) the same regenerative properties. The regeneration showed stability both at short- and at medium-term. However, several studies found better short-term regenerative results using a BG putty combined with platelet rich fibrin (PRF) than PRF alone [
96,
97] or BG than PRF alone [
95]. The different study designs could explain the results. However, the BG considered seem to achieve effective regenerative properties [
92,
93,
94]. The comparison between HA/DFDBA and BG grafting as regenerative properties showed better clinical results with BG grafting than graft covered with bioresorbable membranes [
90,
91], both using OFD surgical technique. On the other hand, Koduru et al. [
98] found similar clinical outcomes compared DFDBA with BG grafts, and Katuri et al. [
85] obtained better results for the DFDBA grafts. However, both the study designs did not cover the grafted material with membranes and, besides, Katuri at al. [
85] used a DFDBA combined in putty form. Within its inherent limitations, the present clinical review shows that BGs have effective properties of periodontal forming. Moreover, BGs could be advantageously grafted without being combined with other compounds or covered by membranes, as necessary for HA graft. This could achieve the periodontal surgical regeneration of the periodontal defect in a further minimally invasive way.
However, to summarize, it is difficult to determine which BG composition can allow the best clinical results or is more promising, because the clinical outcomes also depend on the specific clinical characteristics of periodontal defects, and therefore also on the chosen surgical therapeutic strategy. Moreover, a significant heterogeneity characterized the studies considered.
This entry is adapted from the peer-reviewed paper 10.3390/ma15062194