During pregnancy, cycles of hypoxia and oxidative stress play a key role in the proper development of the fetus. Hypoxia during the first weeks is crucial for placental development, while the increase in oxygen due to the influx of maternal blood stimulates endothelial growth and angiogenesis. However, an imbalance in the number of oxidative molecules due to endogenous or exogenous factors can overwhelm defense systems and lead to excessive production of reactive oxygen species (ROS). Many pregnancy complications, generated by systemic inflammation and placental vasoconstriction, such as preeclampsia (PE), fetal growth restriction (FGR) and preterm birth (PTB), are related to this increase of ROS.
1. Introduction
During the prenatal period, several complications affecting the mother and the fetus can occur, with consequences for their wellbeing. Preeclampsia (PE), a multisystem progressive disease caused by placental and maternal endothelial dysfunction, usually happens late in pregnancy and complicates 2–8% of pregnancies globally
[1]. Moreover, severe and early-onset PE are associated with significant fetal growth restriction (FGR)
[2][3], that refers to the fetus that does not grow to its expected biological potential in utero. FGR affects between 3–9% of pregnancies in developed countries and up to 25% in low-middle income countries
[4]. According to fetal programming theory, adverse events occurring during critical points of fetal development may cause effects on the offspring and predispose them to chronic diseases later in life
[5]. Therefore, placental insufficiency can lead to long-term neurodevelopmental and metabolic diseases, as well as to significant perinatal mortality, especially among prematurely born neonates
[6]. However, not only can the FGR predispose the newborn to chronic diseases throughout its life, but prematurity also affects the normal development and the future health of the newborn
[5]. Preterm birth (PTB), defined as any birth before 37 completed weeks of gestation, is estimated to be about 11% worldwide
[7], i.e., approximately 15 million children are born preterm every year
[8]. Prematurity is related to inflammation and oxidative stress pathways
[9], and is associated with increased mortality. Many survivors face a wide range of lifetime disabilities, which have financial and organizational consequences on already stretched health services. The World Health Organization (WHO) estimates that more than three-quarters of premature babies can be saved with feasible, cost-effective care
[10]. During pregnancy, there is an increase in oxidative stress resulting from placental development, which, under normal conditions, is attenuated by the physiological antioxidant response. The imbalance between oxidative stress and antioxidant response contributes to poor pregnancy outcomes, increasing the risk of PE, FGR and PTB
[11][12][13]. Placental ischemia/hypoxia may contribute to oxidative DNA damage due to the release of reactive ROS into the maternal circulation and can act as an enhancer of FGR. In PE, the redox function is also enhanced compared to normal pregnancies
[14]. Unbalanced oxidative stress and ROS-produced collagen damage to amniotic membranes are involved not only in PTB but also in premature preterm rupture of membranes (PPROM)
[15]. The fetus has to deal with oxidative stress not only in utero, but also at birth, when the newborn has to face a change from an hypoxic environment to a hyperoxic one, which can lead to cytotoxic damage through the creation of ROS and free radicals
[16]. Human breast milk contains agents with antioxidant properties, whose concentrations vary during the different stages of lactation, being higher in colostrum. This could impact the antioxidant status of breast-fed infants
[17]. Therefore, the intake of antioxidants in breastfeeding mothers could influence the antioxidant concentrations in breast milk, and therefore in breastfed newborns. However, studies on the subject are scarce and the choice of the best antioxidant and best dose to improve neonatal oxidative stress damage remains unknown to date. Antioxidant supplementation during pregnancy and breastfeeding could be a promising and cost-effective tool for preventing or treating all these adverse outcomes.
Globally, there is an ongoing scientific effort to find solutions to prevent and ameliorate health complications that both mothers and newborns may suffer during the perinatal period. Antioxidant supplementation during pregnancy and breastfeeding could be a promising and cost-effective tool for preventing or treating all these adverse outcomes.
2. Effects of Antioxidant Intake on Maternal/Neonatal Health
2.1. Curcumin
Curcumin is a polyphenolic substance generally recognized as safe (GRAS), which comes from the rhizomes of
Curcuma longa (turmeric), and has been shown to have antioxidant and anti-inflammatory properties in humans
[18]. In addition, it is emerging as a promising adjuvant in the fight against COVID-19 due to its potential to activate NFE2-related factor-2 (Nrf2) and decrease inflammatory cytokines
[19]. The therapeutic effect of curcumin to counteract certain complications during pregnancy, such as PE and FGR, has been tested in vitro studies, showing an increase in angiogenesis and a decrease in oxidative stress through activation of the Nrf2 signaling pathway
[20][21].
The administration of different doses of this hydrophobic polyphenol by gavage has also been tested in mouse models of fetal restriction by low-protein (LP) diets. It was observed that a dose of 400 mg/kg/day generated significantly greater fetal gain compared to doses of 100 mg/kg/day and both doses significantly increased fetal and placental weights compared to the untreated LP group
[22]. Regardless of dose, in the placenta curcumin reduced levels of oxidative stress marker malondialdehyde (MDA) and of apoptosis to control group levels (without FGR). In contrast, these levels were significantly higher in the untreated LP group. Remarkably, the highest curcumin concentration also restored the percentage of blood sinusoid area in the placenta to control group levels. However, the bioavailability of the antioxidants was not measured in these animals.
2.2. EGCG
Although there is insufficient evidence of the effect of EGCG on PE in humans or in animal models (with no studies in the latter), it has recently been shown that EGCG exerts a protective role against endothelial dysfunction and enhances the anti-angiogenic status in hypoxic trophoblast cells. The protective effect of EGCG appears to be due, in part, to the inhibition of the expression of the high mobility group box 1 (HMGB1), a late inflammatory factor released by trophoblasts during hypoxia that induces endothelial damage
[23]. In addition, it was observed that this antioxidant significantly increased cell viability under hypoxic conditions. These results were dose-dependent, demonstrating the usefulness of EGCG against the main causes of PE, trophoblast apoptosis and endothelial dysfunction
[24][25].
Regarding FGR, although it has been shown in mice that antenatal ECGC can counteract the fetal restriction generated by other reasons, such as alcohol consumption
[26], its effect in humans has not been tested. The postnatal consumption of this antioxidant in mice subjected to FGR decreases fatty acid synthesis through the Ampk/Srebf1 signaling pathway and significantly reduces cholesterol and triglyceride levels in the liver compared to the untreated group
[27]. The relationship between low birth weight and hypertriglyceridemia has been demonstrated
[28][29], so postnatal use of EGCG could help to reduce cardiovascular risk in this population. However, further studies must be conducted.
2.3. Resveratrol
RESV is a polyphenol extracted from fruit, such as grapes and cranberries, and has been reported to be safe for human consumption at doses up to 5 g per day
[30]. In addition, it is able to cross the placenta in both rats and pregnant nonhuman primates
[31][32].
As with EGCG, RESV has been used together with nifedipine in women with severe PE
[33]. The results showed a significantly faster blood pressure decrease than with nifedipine alone, similar to those obtained with EGCG
[34]. RESV, such as EGCG, also significantly increased the time interval before a new hypertensive crisis and none of them produced adverse effects at the neonatal or maternal level. However, the RESV concentration used was lower (50 mg/capsule) compared to EGCG (100 mg/capsule). Co-treatment with RESV in endothelial cells (HUVECs) treated with serum from PE pregnant women restored the levels of heme oxygenase-1 (HO-1) and nitric oxide (NO) markers
[35], key factors for placental vasculature and endothelial protection
[36][37]. Epigallocatechin-3-gallate is the most abundant catechin in green tea and has been extensively studied in numerous clinical studies for the treatment of diabetes, appetite control, weight loss or cognitive improvement
[38][39][40][41]. Moreover, its bioavailability has been tested with different nutritional strategies, facilitating the most appropriate choice based on the purpose of the study
[42].
Despite few human studies with RESV in PE, its effect has been extensively studied in murine models. Dietary supplementation with RESV in a genetic model that mimics the phenotypic characteristics of PE and FGR has shown a significant increase in uterine artery blood flow velocity and fetal weight
[43]. A significant decrease in blood pressure, oxidative stress and apoptosis has also been seen in trophoblasts derived from placentas of PE rats treated with RESV
[44], as well as a significant improvement in placental epithelial characteristics
[45]. However, unlike the previous study, fetus birth weight did not change compared to the non-treated group. Subcutaneous supplementation with RESV through subepidermal patches has also been tested in ewe. Results showed a significant increase in uterine artery blood flow and fetal weight, although maternal RESV treatment had no effect on placental weight
[46]. All of these data present RESV as a promising therapeutic strategy for PE and FGR, although clinical studies in PE and FGR with RESV are currently very limited.
2.4. Melatonin
Melatonin, synthesized mainly in the pineal gland, can easily cross the placenta and exert its antioxidant action, regulate cell proliferation in the fetus, and maintain pregnancy
[47][48]. A recent meta-analysis showed that its concentration is significantly lower in women with PE and its levels correlate with the severity of the disease, being significantly lower in severe PE than mild PE
[49]. Likewise, in the case of placental insufficiency, melatonin 1A and 1B receptors are significantly less expressed in the placental tissue of mothers of FGR fetuses
[50]. In addition, melatonin and placental growth factor (PLGF) in the umbilical blood were significantly lower in this group compared to normal pregnancies
[51][52].
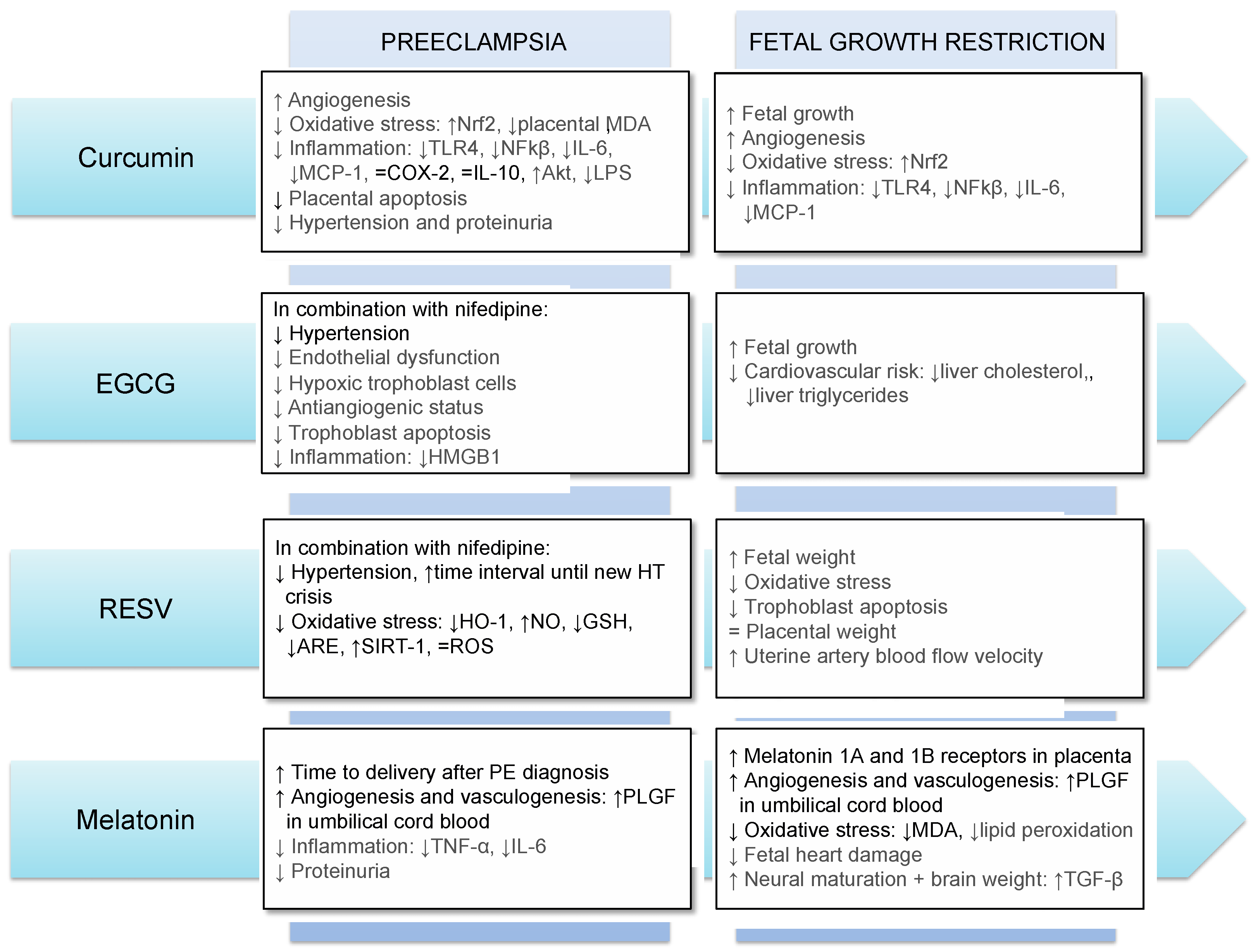
The main results obtained so far regarding the use of antioxidants in FGR and PE in humans and animals are summarized in Figure 1.
Figure 1. Main effects of the use of curcumin, EGCG, resveratrol and melatonin in preeclampsia and fetal growth restriction. Results in gray and black refer to those obtained in animal models and human, respectively. Abbreviations: Akt: Protein kinase B; ARE: antioxidant response element; COX-2: Cyclooxygenase-2; GSH: glutathione; HMGB1: high-mobility group box 1; HO-1: heme oxygenase-1; HT: hypertension; IL-6: interleukin-6; IL-10: interleukin-10; LPS: lipopolysaccharides; MCP-1: monocyte chemoattractant protein-1; MDA: malondialdehyde; NFkβ: nuclear factor κB; NO: nitric oxide; Nrf2: NFE2-related factor-2; RESV: resveratrol; ROS: reactive oxygen species; SIRT-1: sirtuin-1; TGF-β: transforming growth factor-beta; TLR4: Toll Like Receptor 4; TNF-α: tumor necrosis factor-alpha; PLGF: placental growth factor; ↓: decrease; ↑: increase; =: no difference.
This entry is adapted from the peer-reviewed paper 10.3390/antiox11040648