Transfersomes are elastic in nature, which can deform and squeeze themselves as an intact vesicle through narrow pores that are significantly smaller than its size. Encapsulating the drugs in transfersomes are one of the potential approaches to overcome the barrier function of the skin’s outermost layer. They have a bilayered structure that facilitates the encapsulation of lipophilic and hydrophilic, as well as amphiphilic, drug with higher permeation efficiencies compared to conventional liposomes.
- transfersomes
- nanoencapsulation
- transdermal drug delivery
1. Introduction
An efficacious, successful therapeutic treatment cannot be achieved in most cases, often due to many reasons, such as the occurrence of hepatic first-pass metabolism, adverse side effects, the rejection of invasive treatments and poor patient compliance[1]. Therefore, several drug delivery systems have been developed and studied over the past decades to overcome these problems. One promising approach is the use of transdermal delivery systems, as they are minimally invasive methods without first-pass effects. However, the barrier function of the skin that prevents or dampens the transdermal delivery of therapeutic agents has to be addressed[2][3].
A new type of carrier system—namely, transfersomes—were introduced by Cevc et al. in the 1990s. Transfersomes are composed of phospholipids and edge activator (EA), which is a membrane-softening agent (such as Tween 80, Span 80 and sodium cholate) that facilitates the ultra-deformable property of the transfersomes. When transfersomes reach the skin pores, they are capable of changing their membrane flexibility and passing through the skin pores spontaneously. This is the so-called self-optimizing deformability[4]. Moreover, transferomes are extremely deformable; therefore, they easily cross even the very narrow pores[5]. These self-optimizing, highly deformable lipid aggregates were successfully used in extensive preclinical tests and diverse arrays of phase I and phase II clinical trials, as well as for the transcutaneous delivery of peptides and proteins and the sustain release of desired therapeutic agents[6][7]. A number of transfersomes-based formulations are currently being assessed at different stages of clinical trials. For example, the study of the safety and efficacy of ketoprofen incorporated in transfersomes (Diractin®) for the treatment of osteoarthritis of the knees was carried out under a phase III clinical trial.
2. Transfersomes
Transferosomes are vesicular carrier systems that are specially designed to have at least one inner aqueous compartment that is enclosed by a lipid bilayer, together with an edge activator (Figure 1)[4].
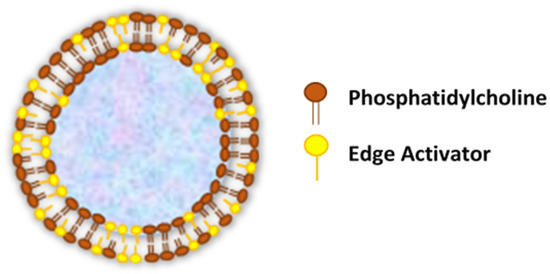
Figure 1. Structure of transfersomes.
This aqueous core surrounded by a lipid bilayer makes ultra-deformable vesicles having both self-optimizing and self-regulating capabilities[8]. In accordance with that, transfersomes are elastic in nature and can thereby deform and squeeze themselves as intact vesicles without a measurable loss through narrow pores or constrictions of the skin that are significantly smaller than the vesicle size[9][10].
In contrast to conventional liposomes, which are comprised of natural (such as egg phosphatidylcholine—EPC and soybean phosphatidylcholine—SPC) or synthetic (such as dimyristoyl phosphatidylcholine—DMPC, dipalmitoyl phosphatidylcholine—DPPC and dipalmitoyl phosphatidyl glycerol—DPPG) phospholipids[11], the modified liposomal vesicular system (transfersomes) is composed of the phospholipid component and single-chain surfactant as an edge activator[12].
Edge activators (EAs) function in an exceptional manner as membrane-destabilizing factors to increase the deformability of vesicle membranes and, when combined in a proper ratio with an appropriate lipid, gives the optimal mixture, enabling the transfersomes to become deformable, as well as ultra-flexible, which results in a higher permeation capability[13][14]. Therefore, transfersomes overcome the major drawbacks of conventional liposomes and penetrate pores that are much smaller than their own diameters. Furthermore, the transfersomes maintained their diameters against fragmentation, even after penetration through the smaller-sized pores. Due to the usage of EAs in the transfersomal formulation, it has achieved an enhanced performance compared to the conventional liposomes[13][14].
The EAs used in transfersomal formulations can also facilitate the solubilization of hydrophobic drugs, thereby increasing the drug entrapment efficiency of the formulations [15][16][17]. Moreover, the EAs have the potential to solubilize and fluidize the skin lipids, resulting in skin permeation enhancements [18][19]. The effect of EAs associated in skin permeations depends on their types and concentrations. Surfactants are one of many different compounds that act as edge activators and penetration enhancers[20]. They are known to be amphiphilic molecules that consist of a lipophilic alkyl chain that is connected to a hydrophilic head group[21]. Generally, rather than cationic surfactants, anionic surfactants are furthermore effective in enhancing the skin penetration, and the critical micelle concentration is also lower, whereas nonionic surfactants with an uncharged polar head group are better-tolerated than cationic and anionic surfactants[20].
Advantages of Transfersomes as Vesicle-based Transdermal Drug Delivery Systems [2][11][22][23]:
- Transfersomes carriers are composed of hydrophilic and hydrophobic moieties, which result in becoming a unique drug carrier system that can deliver therapeutic agents with wide range of solubility.
- Transfersomes are able to squeeze themselves through constrictions of the skin barrier that are very narrow, such as 5 to 10 times less than the vesicle diameter, owing to their ultra-deformability and elastic properties.
- High vesicle deformability facilitates the transport of drugs across the skin without any measurable loss in intact vesicles and can be used for both topical, as well as systemic, treatments.
- Transfersomes carriers are very versatile and efficient in accommodating a variety of agents nearly independent of their size, structure, molecular weight or polarity.
- They are made up of natural phospholipids and EAs, therefore promisingly biocompatible and biodegradable.
- Transfersomes can be used for the delivery of various active compounds, including proteins and peptides, insulin, corticosteroids, interferons, anesthetics, NSAIDs, anticancer drugs and herbal drugs.
- Transfersomes are an obvious choice for achieving a sustained drug release, as well as a predictable and extended duration of activity.
- They are capable of increasing the transdermal flux and improving the site specificity of bioactive agents.
- Avoiding the first-pass metabolism, which is a major drawback in oral drug administration, and result in optimized bioavailability of the drug.
- Minimize the undesirable side effects of the drug, as well as protect the drug from metabolic degradation; moreover, the utility of short half-life drugs.
- In most of the cases, a relatively high entrapment efficiency (EE) of nearly 90% of the lipophilic drug can be achieved by transfersomes. For transfersome formulations of diclofenac diethylamine (DDEA) and curcumin (CRM), the maximum entrapment efficiency achieved was over 90% for both DDEA and CRM transfersomes. Nevertheless, the entrapment efficiency can variate due to various reasons, as, when the lipid concentration was more, a high entrapment efficiency could be observed. The EE decreases when the surfactant concentration increases above certain concentrations due to the formation of mixed micelles. According to the literature, in case of a low EE, the lipophilic drug encapsulation could be enhanced by incorporating a surfactant with a low HLB (hydrophilic-lipophilic balance) value. It has been identified as a fact that transfersomes show a distinctive property of the very high encapsulation of lipophilic drugs.
- They have the advantage of being made from pharmaceutically acceptable ingredients using standard methods but need to be designed and optimized on a case-by-case basis.
- Due to a short and simple production procedure, it is easy to scale-up.
Limitations of Transfersomes[2][11][24]:
- Transfersomes are considered as chemically unstable due to their tendency to oxidative degradation. The oxidation of transfersomes can be significantly decreased when the aqueous media is degassed and purged with inert gases, such as nitrogen and argon[25]. Storage at a low temperature and protection from light will also reduce the chance of oxidation[26]. Post-preparation processing, such as freeze-drying and spray-drying, can improve the storage stability of transfersomes[27].
- Another obstacle of utilizing transfersomes as a drug delivery system is the difficulty to achieve the purity of natural phospholipids. Therefore, synthetic phospholipids could be used as alternatives[28].
- The expensiveness of transfersomal formulations is associated with the raw materials used in lipid excipients, as well as the expensive equipment needed to increase manufacturing. Hence, the widely used lipid component is phosphatidylcholine, because it is relatively low in cost[29].
3. Mechanism of Action
Vesicles are known as colloidal particles, which are an aqueous compartment enclosed by a concentric bilayer that are made-up of amphiphilic molecules. They are very useful as vesicular drug delivery systems, which transport hydrophilic drugs encapsulated in the inner aqueous compartment, whereas hydrophobic drugs are entrapped within the lipid bilayer [30]. With regard to transfersomes, they are highly deformable (ultra-flexible) and self-optimizing novel drug carrier vesicles, in which their passage across the skin is mainly associated with the transfersomes’ membrane flexibility, hydrophilicity and the ability to maintain the vesicle’s integrity (Figure 2)[31][32].
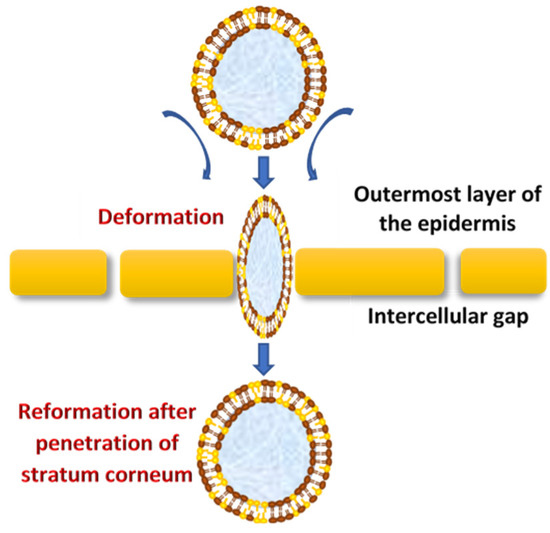
Figure 2. The mechanism of action of transfersomes.
They efficiently penetrate through the intact skin if applied under nonocclusive conditions; this specific nonocclusive state of the skin is required mainly to initiate a transepidermal osmotic gradient across the skin[1][33].
The transdermal water activity difference, which originates due to the natural transdermal gradient, creates a significantly strong force that acts upon the skin through transfersomes vesicles, which enforce the widening of intercellular junctions with the lowest resistance and thereby generate transcutaneous channels 20–30 nm in width. These created channels allow the transfer of ultra-deformable, slimed transfersomes across the skin with respect to the hydration gradient[5]. Moreover, the osmotic gradient develops as a result of evaporation of the skin surface water due to body heat, which exerts its action as the driving force to facilitate the flexible transport across the skin to deliver therapeutic agents from the site of application to the target area for local or systemic treatments in effective therapeutic concentrations and minimum systemic toxicity[8]. Transfersomes demonstrate a higher permeation efficiency (through small skin channels) compared to conventional liposomes but have a similar bilayered structure that facilitates the encapsulation of lipophilic and hydrophilic, as well as amphiphilic, drugs[34]. Transfersomes vary from liposomes, primarily due to their softer, better adjustable and ultra-deformable artificial membranes. Interdependency of the local composition, as well as the shape of the lipid bilayer, makes the vesicles both self-optimizing and self-regulating. This property enables the transfersomes vesicles to cross numerous transport barriers efficiently.
4. Composition of Transfersomes
Transfersomes are generally composed of
- firstly, the main ingredient, an amphipathic ingredient (e.g., soy phosphatidylcholine, egg phosphatidylcholine, etc.) that can be a mixture of lipids, which are the vesicle-forming components that create the lipid bilayer[27][35].
- secondly, 10–25% surfactants/edge activators; the most commonly used edge activators in transfersome preparations are surfactants as sodium cholates; sodium deoxycholate; Tweens and Spans (Tween 20, Tween 60, Tween 80, Span 60, Span 65 and Span 80) and dipotassium glycyrrhizinate, which are biocompatible bilayer-softening compounds that increase the vesicles’ bilayer flexibility and improve the permeability [3][6][35][36][37].
about 3–10% alcohol, as the solvent and, finally, hydrating medium consist with either water or a saline phosphate buffer (pH 6.5–7)[38][39].
5. Factors Affecting Properties of Transfersomes
In the process of obtaining an optimized formulation of transfersomes, there are number of process variables that could affect the properties of the transfersomes. These variables basically involve the manufacturing of transfersomal formulations, which are identified as follows:
Effect of Phospholipids: Edge Activator Ratio
The phospholipid: Edge activator (lecithin:surfactant) should be an optimized ratio due to the fact that this greatly affects the entrapment efficiency, vesicle size and permeation ability. In general, it has been reported that the EE could be reduced due to the presence of a higher surfactant concentration. This may be due to the result of increased vesicles’ membrane permeability because of the arrangement of surfactant molecules within the vesicular lipid bilayer structure, which could generate pores within the vesicular membrane and lead to an increased fluidity and prompt the leakage of the entrapped drug[23]. A further increase in the edge activator content may lead to pore formation in the bilayer and a reduced permeation ability of the vesicles[33], whereas the incorporation of low concentrations of surfactants may result in growth of the vesicle size. In addition, the decrease in vesicles size at high phospholipid concentrations has been reported in various studies[40][41].
Effect of Various Solvents
Various solvents such as ethanol or methanol are used. Selection of the appropriate solvent depends on the solubility of all the formulation ingredients in the solvent and their compatibility with the solvent. Preferably, all the excipients, including the drug, should completely dissolve in the solvent and should obtain a clear transparent solution to produce a better film-forming ability and good stability after hydration[42]. Solvents used in the formulation can also exert their function as penetration enhancers that improve drug flux through the membrane. According to Williams and Barry (2004), ethanol was used in various studies to enhance the flux of hydrocortisone, 5-fluorouracil, estradiol and levonorgestrel through rat skin [43]. For an example, ethanol increases the permeation through different mechanisms, such as increasing the drug solubility in vesicles by acting as a solvent, moreover permeating into the stratum corneum and altering the solubility properties of the respective tissue and, consequently, improving the drug partitioning into the membrane. Increasing the ethanol concentration in the formulation may result in a decrease in the %EE, which could be attributed to the increased permeability of the vesicular phospholipid bilayer. This may promote the consequent leakage of the encapsulated drug[44].
Effect of Various Edge Activators (Surfactants)
Deformability, as well as the entrapment efficiency of transfersome vesicles, are affected by the type of edge activators used in their formulations. This could be due to the difference in the chemical structure of the EA[34]. Generally, the vesicle size decreases by increasing the surfactant concentration, the hydrophilicity of the surfactant head group, carbon chain length and the hydrophilic lipophilic balance (HLB). The three surfactants, including tween 80, span 80 and sodium deoxycholate, were used to prepare the transfersomes, and a reduction of the vesicle size was found when the higher surfactant concentration used. This might be due to the fact that the high surfactant concentrations (more than 15%) induce micelle formation rather than vesicle formation[45]. A small polydispersity index (PDI) was reported with the higher surfactant concentration. A small PDI is responsible for consistent size distribution, which is thought to be an important factor for the reduction of interfacial tension and provides a homogeneous formulation. Additionally, an increased surfactant concentration may lead to an increase in charge of the vesicles, which results in a reduction of vesicle aggregation and enhances the stability of the system. In addition, surfactant properties are one of the properties that are responsible for the entrapment efficiency of the vesicles, as, for an example, the entrapment of a lipophilic drug would be enhanced with the use of a surfactant with a low HLB value. Moreover, it has been reported that higher surfactant concentrations will increase the formation of the vesicle number, which leads to a higher volume of the hydrophobic bilayer domain that is available for the entrapment of hydrophobic drugs. However, if the amount of lipophilic drug exceeds the vesicular loading capacity, it may disrupt the vesicular membrane, leading to drug leakage, lowering the entrapment efficiency and skin permeation ability[46]. Furthermore, the membrane permeability of vesicles depends on the carbon chain length and transition temperature of the surfactant. The optimum amount of surfactant used in the formulation depends on the packing density of the phospholipid used and the surfactant-phospholipid interaction[23]. The presence of surfactants can have an impact on the permeation property of transfersomes. According to a study by Cipolla et al. (2014), the amount of drug (ciprofloxacin) released was dependent on the concentration, as well as the type of surfactant used, and using Tween 80 significantly increased the release[47].
Effect of the Hydration Medium
The hydrating medium may consist of either water or saline phosphate buffer (pH 6.5–7). The pH level of the formulation should be suitable to achieve a balance between both the formulation properties and biological applications, as well as the route of administration. The lipid bilayer of transfersomes mimics the phospholipid layer of the cell membrane, and only unionized drugs remain membrane-bound to the phospholipid bilayer and penetrate through the intracellular route[48][49].
It is important to use the suitable pH of the hydration medium, which keeps the drug unionized to increase the entrapment and permeation of the drug.
6. Conclusions
Transfersomes are ultra-deformable carriers that facilitate the delivery of a diverse array of drug molecules across the skin barrier with superior efficacy compared to the conventional vesicular systems. The osmotic gradient is the main driving force for the transport of transfersomes into the deeper skin layers. Importantly, transferosomes are specifically designed vesicular systems that need to be optimized in accordance with individual cases of drugs of interest to achieve the most effective formulations and ultimate pharmacological responses. Further scientific studies associated with transfersomes may lead to novel promising therapeutic approaches against many types of diseases.
This entry is adapted from the peer-reviewed paper 10.3390/pharmaceutics12090855
References
- Chaurasiya, P.; Ganju, E.; Upmanyu, N.; Ray, S.K.; Jain, P. Transfersomes: A novel technique for transdermal drug delivery. J. Drug Deliv. Ther. 2019, 9, 279–285.
- Modi, C.; Bharadia, P. Transfersomes: New dominants for transdermal drug delivery. Am. J. PharmTech. Res. 2012, 2, 71–91.
- Jain, A.K.; Kumar, F. Transfersomes: Ultradeformable vesicles for transdermal drug delivery. Asian J. Biomater. Res. 2017, 3, 1–13.
- Rai, S.; Pandey, V.; Rai, G. Transfersomes as versatile and flexible nano-vesicular carriers in skin cancer therapy: The state of the art. Nano Rev. Exp. 2017, 8, 1325708.
- Cevc, G.; Schatzlein, A.G.; Richardsen, H. Ultradeformable lipid vesicles can penetrate the skin and other semi-permeable barriers unfragmented. Evidence from double label CLSM experiments and direct size measurements. Biochim. et Biophys. Acta (BBA)-Biomembr. 2002, 1564, 21–30.
- Rajan, R.; Jose, S.; Mukund, V.P.B.; Vasudevan, D.T. Transferosomes—A vesicular transdermal delivery system for enhanced drug permeation. J. Adv. Pharm. Technol. Res. 2011, 2, 138–143.
- Cevc, G. Lipid vesicles and other colloids as drug carriers on the skin. Adv. Drug Deliv. Rev. 2004, 56, 675–711.
- Walve, J.R.; Bakliwal, S.R.; Rane, B.R.; Pawar, S.P. Transfersomes: A surrogated carrier for transdermal drug delivery system. Int. J. Appl. Biol. Pharm. Technol. 2011, 2, 204–213.
- Sivannarayana, P.; Rani, A.P.; Saikishore, V.; VenuBabu, C.; SriRekha, V. Transfersomes: Ultra deformable vesicular carrier systems in transdermal drug delivery system. Res. J. Pharm. Dos. Forms Technol. 2012, 4, 243–255.
- Sachan, R.; Parashar, T.; Soniya, S.V.; Singh, G.; Tyagi, S.; Patel, C.; Gupta, A. Drug carrier transfersomes: A novel tool for transdermal drug delivery system. Int. J. Res. Dev. Pharm. Life Sci. 2013, 2, 309–316.
- Li, J.; Wang, X.; Zhang, T.; Wang, C.; Huang, Z.; Luo, X.; Deng, Y. A review on phospholipids and their main applications in drug delivery systems. Asian J. Pharm. Sci. 2015, 10, 81–98.
- Bhasin, B.; Londhe, V.Y. An overview of transfersomal drug delivery. Int. J. Pharm. Sci. Res. 2018, 9, 2175–2184.
- Lei, W.; Yu, C.; Lin, H.; Zhou, X. Development of tacrolimus-loaded transfersomes for deeper skin penetration enhancement and therapeutic effect improvement in vivo. Asian J. Pharm. Sci. 2013, 8, 336–345.
- Pandey, A. Role of surfactants as penetration enhancer in transdermal drug delivery system. J. Mol. Pharm. Org. Process. Res. 2014, 2, 1–10.
- Duangjit, S.; Opanasopit, P.; Rojanarata, T.; Ngawhirunpat, T. Characterization and in vitro skin permeation of meloxicam-loaded Liposomes versus Transfersomes. J. Drug Deliv. 2010, 2011, 1–9.
- Aggarwal, N.; Goindi, S. Preparation and evaluation of antifungal efficacy of griseofulvin loaded deformable membrane vesicles in optimized guinea pig model of Microsporum canis—Dermatophytosis. Int. J. Pharm. 2012, 437, 277–287.
- Chen, J.; Lu, W.-L.; Gu, W.; Lu, S.-S.; Chen, Z.-P.; Cai, B.-C. Skin permeation behavior of elastic liposomes: Role of formulation ingredients. Expert Opin. Drug Deliv. 2013, 10, 845–856.
- Duangjit, S.; Opanasopit, P.; Rojanarata, T.; Ngawhirunpat, T. Effect of edge activator on characteristic and in vitro skin permeation of meloxicam loaded in elastic liposomes. Adv. Mater. Res. 2011, 194, 537–540.
- Jacob, L.; Anoop, K.R. A review on surfactants as edge activators in ultradeformable vesicles for enhanced skin delivery. Int. J. Pharma Bio Sci. 2013, 4, 337–344.
- Kim, B.; Cho, H.-E.; Moon, S.H.; Ahn, H.-J.; Bae, S.; Cho, H.-D.; An, S. Transdermal delivery systems in cosmetics. Biomed. Dermatol. 2020, 4, 1–12.
- Kumar, G.P.; Rajeshwarrao, P. Nonionic surfactant vesicular systems for effective drug delivery—An overview. Acta Pharm. Sin. B 2011, 1, 208–219.
- Moawad, F.A.; Ali, A.A.; Salem, H.F. Nanotransfersomes-loaded thermosensitive in situ gel as a rectal delivery system of tizanidine HCl: Preparation, in vitro and in vivo performance. Drug Deliv. 2017, 24, 252–260.
- Bnyan, R.; Khan, I.; Ehtezazi, T.; Saleem, I.; Gordon, S.; Neill, F.O.; Roberts, M. Surfactant effects on lipid-based vesicles properties. J. Pharm. Sci. 2018, 107, 1237–1246.
- Kumar, A. Transferosome: A recent approach for transdermal drug delivery. J. Drug Deliv. Ther. 2018, 8, 100–104.
- MirAfzali, Z.; Thompson, C.S.; Tallua, K. Chapter 13—Application of liposomes in the food industry. In Microencapsulation in the Food Industry: A Practical Implementation Guide; Academic Press: Cambridge, MA, USA, 2014; pp. 139–150.
- Grit, M.; Crommelin, D.J. Chemical stability of liposomes: Implications for their physical stability. Chem. Phys. Lipids 1993, 64, 3–18.
- Iskandarsyah; Rahmi, A.D.; Pangesti, D.M. Comparison of the characteristics of transfersomes and protransfersomes containing azelaic acid. J. Young-Pharm. 2018, 10, S11–S15.
- Van Hoogevest, P.; Wendel, A. The use of natural and synthetic phospholipids as pharmaceutical excipients. Eur. J. Lipid Sci. Technol. 2014, 116, 1088–1107.
- Yadav, D.; Sandeep, K.; Pandey, D.; Dutta, R.K. Liposomes for drug delivery. J. Biotechnol. Biomater. 2017, 7, 1–8.
- Mathur, M. Approaches for improving the pharmacological and pharmacokinetics properties of herbal drugs. Int. Res. J. Pharm. Appl. Sci. 2013, 3, 40–50.
- Jadupati, M.; Kumar, N.A. Transferosome: An opportunistic carrier for transdermal drug delivery system. Int. Res. J. Pharm. 2012, 3, 35–38.
- Cevc, G.; Blume, G.; Schätzlein, A.; Gebauer, D.; Paul, A. The skin: A pathway for systemic treatment with patches and lipid-based agent carriers. Adv. Drug Deliv. Rev. 1996, 18, 349–378.
- Cevc, G. Transdermal drug delivery of insulin with ultradeformable carriers. Clin. Pharmacokinet. 2003, 42, 461–474.
- Chauhan, P.; Tyagi, B.K. Herbal novel drug delivery systems and transfersomes. J. Drug Deliv. Ther. 2018, 8, 162–168.
- Jiang, T.; Wang, T.; Li, T.; Ma, Y.; Shen, S.; He, B.; Mo, R. Enhanced transdermal drug delivery by transfersome-embedded oligopeptide hydrogel for topical chemotherapy of melanoma. ACS Nano 2018, 12, 9693–9701.
- Kotla, N.G.; Chandrasekar, B.; Rooney, P.; Sivaraman, G.; Larrañaga, A.; Krishna, K.V.; Pandit, A.; Rochev, Y. Biomimetic lipid-based nanosystems for enhanced dermal delivery of drugs and bioactive agents. ACS Biomater. Sci. Eng. 2017, 3, 1262–1272.
- Ascenso, A.; Batista, C.; Cardoso, P.; Mendes, T.; Praça, F.G.; Bentley, M.V.L.B.; Raposo, S.; Simões, S. Development, characterization, and skin delivery studies of related ultradeformable vesicles: Transfersomes, ethosomes, and transethosomes. Int. J. Nanomed. 2015, 10, 5837–5851.
- Pawar, A.Y.; Jadhav, K.R.; Chaudhari, L.H. Transfersome: A novel technique which improves transdermal permeability. Asian J. Pharm. 2016, 10, 425–436.
- Garg, V.; Singh, H.; Bimbrawh, S.; Singh, S.K.; Gulati, M.; Vaidya, Y.; Kaur, P. Ethosomes and transfersomes: Principles, perspectives and practices. Curr. Drug Deliv. 2016, 14, 613–633.
- Vasanth, S.; Dubey, A.; Ravi, G.S.; Lewis, S.A.; Ghate, V.M.; El-Zahaby, S.A.; Hebbar, S. Development and investigation of vitamin c-enriched adapalene-loaded transfersome gel: A collegial approach for the treatment of acne vulgaris. AAPS Pharm. Sci. Tech. 2020, 21, 61.
- Qushawy, M.; Nasr, A.; Abd-Alhaseeb, M.; Swidan, S. Design, optimization and characterization of a transfersomal gel using miconazole nitrate for the treatment of candida skin infections. Pharmaceutics 2018, 10, 26.
- El Zaafarany, G.M.; Awad, G.A.S.; Holayel, S.M.; Mortada, N. Role of edge activators and surface charge in developing ultradeformable vesicles with enhanced skin delivery. Int. J. Pharm. 2010, 397, 164–172.
- Williams, A.C.; Barry, B.W. Penetration enhancers. Adv. Drug Deliv. Rev. 2004, 56, 603–618.
- Shamma, R.N.; Elsayed, I. Transfersomal lyophilized gel of buspirone HCl: Formulation, evaluation and statistical optimization. J. Liposome Res. 2013, 23, 244–254.
- Jain, S.K.; Jain, P.; Umamaheshwari, R.B.; Jain, N.K. Transfersomes—A novel vesicular carrier for enhanced transdermal delivery: Development, characterization, and performance evaluation. Drug Dev. Ind. Pharm. 2003, 29, 1013–1026.
- Balata, G.F.; Faisal, M.M.; Elghamry, H.A.; Sabry, S.A. Preparation and characterization of ivabradine HCl transfersomes for enhanced transdermal delivery. J. Drug Deliv. Sci. Technol. 2020, 60, 101921.
- Cipolla, D.; Wu, H.; Gonda, I.; Eastman, S.; Redelmeier, T.; Chan, H.-K. Modifying the release properties of liposomes toward personalized medicine. J. Pharm. Sci. 2014, 103, 1851–1862.
- Dudhipala, N.; Mohammed, R.P.; Youssef, A.A.A.; Banala, N. Effect of lipid and edge activator concentration on development of Aceclofenac loaded transfersomes gel for transdermal application: In vitro and ex vivo skin permeation. Drug Dev. Ind. Pharm. 2020, 46, 1–28.
- N’Da, D.D. Prodrug strategies for enhancing the percutaneous absorption of drugs. Molecules 2014, 19, 20780–20807.
