Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
The endocannabinoid system (ECS) is an essential signaling system in mammal physiology regulating several biological and disease conditions. ECS’s three main components are (i) cannabinoids receptors (CBR) CBR-1 and CBR-2; (ii) signaling molecules that are lipid-based termed the “endocannabinoids” (EC), endogenous ligands of the CBRs; and (iii) enzymes responsible for synthesizing and degrading ECs. ECS regulates multiple physiological processes, such as brain plasticity and neuronal development, cell death, inflammation, sleep, appetite, pain, and anxiety.
- medical cannabis
- endocannabinoids
- phytocannabinoids
1. Introduction
“Endocannabinoids” (EC) are comprised of highly lipophilic ligands derived from the fatty acid-arachidonic acid (AA), and the first identified EC is called anandamide, also known as N-arachidonoylethanolamine (AEA) and 2-arachidonoyl glycerol (2-AG) [1][2]. Initially isolated from the porcine brain, anandamide is a partial agonist with CBR-1 and CBR-2. Next, 2-AG was isolated from canine intestines as a full agonist with CBR-1 and CBR-2 [3][4][5]. Further putative ECs have been discovered more recently. For example, the EC 2-arachidonoyl glycerol ether (2-AGE, Noladin ether) binds to CBR-1, and CBR-2, showing agonistic properties to both receptors and partial agonistic characters to the TRPV1 channel (Figure 1) [6][7]. The first discovered antagonist for CBR (CBR-1) was Virodhamine (O-AEA), even though its physiological role has yet to be fully understood (Figure 1). [8]. At a later stage, the enzymes, fatty acid amide hydrolase (FAAH) and monoacylglycerol lipase (MAGLs), responsible for the degradation of ECs, were further discovered and characterized [9].
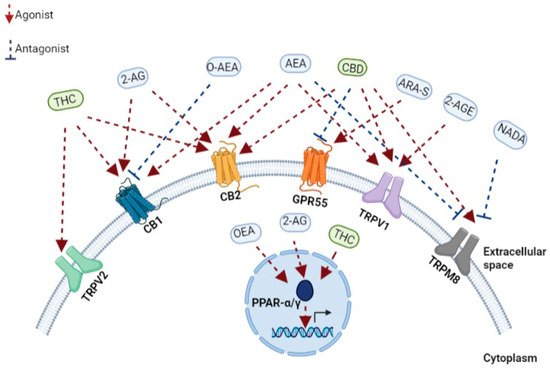
Figure 1. A schematic representation of the main components of the endocannabinoid system. Receptors, endogenous cannabinoids, and cannabis-based components. THC, tetrahydrocannabinol; CBD, cannabidiol; 2-AG, 2-arachidonoylglycerol; O-AEA, virodhamine; AEA, anandamide; ARA-S, N-arachidonoyl serine; 2-AGE, 2-arachidonoyl glycerol ether; NADA, N-arachidonoyl dopamine; TRPV2, transient receptor potential cation channel subfamily V member 2; CB1, cannabinoid receptor 1; CB2, cannabinoid receptor 2; GPR55, G protein-coupled receptor 55; TRPV1, transient receptor potential cation channel subfamily V member 1; TRPM8, transient receptor potential cation channel melastatin 8; PPAR-α, peroxisome proliferator-activated receptor-alpha; PPAR-γ, peroxisome proliferator-activated receptor-gamma.
2. ECS and CBRs—Regulating Immune Functions
The effects of the ECs are primarily mediated by CBR-1 and CBR-2 receptors when the impact of other recently discovered cannabinoid receptors, such as peroxisome proliferator-activated receptors (PPARs) and transient receptor potential channels (TRP channels), are not yet fully interpreted [10]. CBR-1 and CBR-2 are G-protein-coupled receptors; their activation inhibits adenylyl cyclase and specific voltage-dependent calcium channels and activates mitogen-activated protein kinase (MAPK) [10]. Therefore, the activation of CBR’s has diverse consequences on cellular physiology, synaptic function gene transcription [10].
In 1988, using a radiolabelled ligand specifically binding to the CBR, the CBR-1 receptor was discovered [11]. CBR-1 is primarily expressed in the mammalian brain, including the cortex, basal ganglia, hippocampus, and cerebellum [12]. The widespread distribution of the CBR-1 is associated with regulating various cognitive and neuronal functions, ranging from memory, mood, appetite, and sensory responses [13]. Nonetheless, CBR-1 is also expressed at low levels in peripheral tissues and cells [14], and its expression can be found in the adrenal gland, heart, liver, lung, and other peripheral tissues [15][16]. The activation of the presynaptic CBR-1 inhibits the release of neurotransmitters from the synapses by decreasing calcium conductance and increasing potassium conductance [17].
In contrast to CBR-1, the CBR-2 receptor is expressed at a low level in the central nervous system (CNS). CBR-2 is primarily expressed in the spleen, tonsils, thymus, and other tissues and is believed to be responsible mainly for regulating the immune system [14][18]. CBR-2 mRNA expression differs between immune system cells and is expressed at the following hierarchy: B cells > natural killer (NK) cells > polymorphonuclear (PMN) > neutrophils > CD8+ T cells > monocytes > CD4+ T cells [14]. The observation that peripheral blood mononuclear cells (PBMC) from marijuana smokers elevate the expression of CBR receptors compared to healthy donors [19] suggested that CBR’s expression levels on immune cells may be sensitive and adjusted by exposure to phytocannabinoids. On the same line, cytokines could also regulate the CBRs expression [20], while transforming growth factor-beta (TGF-β) regulated CBR-2 expression in human peripheral blood lymphocytes (PBL) via a negative autocrine regulatory loop and IFN-γ produced by T helper 1 (Th1) and NK cells increase the expression of CBR-2 in rat macrophages in neuropathic pain or multiple sclerosis (MS) animal models [21][22]. Accordingly, CBR-1-deficient mice cannot develop lipopolysaccharide-evoked fever, have a lower expression of Toll-like receptor 4 (TLR4) than wild-type (WT) mice, and their peritoneal macrophages do not secrete pro-inflammatory cytokines in response to lipopolysaccharide (LPS) [23]. Conversely, CBR-2 expression protects against acute experimental sepsis when CB2-R deletion (CB2R(−/−)) sensitizes mice to LPS induced death, and the CBR-2 agonist, GW405833, could significantly extend their survival [24].
A few other receptors have recently been identified and characterized as cannabinoids interacting and binding receptors. PPARs are a family of nuclear receptors that include PPARα, PPARγ, and PPARβ/δ, acting as transcription factors primarily involved in the transcriptional regulation of genes linked with inflammation and metabolism [25]. The increased transcriptional activity of PPARα after binding the endocannabinoids 2-AG and oleoyl ethanolamide (OEA) has been shown to regulate genes associated with metabolism and anti-inflammatory effects (Figure 1) [26]. Similarly, different phytocannabinoids can bind, interact, and regulate PPAR activity when natural and synthetic phytocannabinoids, including THC, CBD, cannabimovone (CBM), and quinone derivatives, are all characterized as PPARγ agonists (Figure 1) [26][27].
TRP channels, known as modulators of ion entry, mediate various neuronal signals controlling the sensation of pressure, temperature, and smell and are also regulated by cannabinoids [28]. In recent years, it has come to light that the TRP channel also retains a functional extra-neuronal expression in immune and epithelial cells with important implications for mucosal immunology. For example, TRP channels maintain intracellular calcium homeostasis, regulating various immunological functions, such as producing and releasing inflammatory mediators, phagocytosis, or even immune-cell migration [29]. Correspondingly with the role of ECS in immunity, AEA was identified as an endogenous agonist of TRPV1 [30], while later, N-arachidonoyl dopamine (NADA) and AEA were also characterized as TRPM8 antagonists (Figure 1) [31]. Furthermore, phytocannabinoids can bind and regulate TRPV channels when CBD is a potent agonist at TRPV1 and TRPM8 (Figure 1) [32], while delta9-THC mainly interacts with TRPV2 but also moderately modulates with TRPV3, TRPV4, TRPA1, and TRPM8 activities (Figure 1) [28][32]. Thus, understanding EC’s mechanism in influencing these channels is essential for various immunological and therapeutic processes, such as inflammation and its resolution and host immunity [28].
The G protein-coupled receptors (GPCRs) are prevalent and popular pharmaceutical targets for drug design, representing one of the largest and most versatile cell surface receptors family [33]. The activation of GPCRs is achieved by a diverse range of ligands, including chemokines, lipids, vitamins, hormones, protein, peptides, and neurotransmitters [34]. Therefore, GPCRs are involved in many fundamental cellular and physiological processes, such as the ability to sense light, taste and smell, metabolism, endocrine, and exocrine secretion [35].
In recent years, GPCRs have been found to play an important role also in the immune system [35]. GPCRs, such as GPR18, GPR55, and GPR119, regulate signals associated with cell proliferation and migration [36]. Moreover, GPR18 is expressed on human leukocytes and is believed to be responsible for regulating immune system cells during inflammation [37]. The endocannabinoid N-arachidonoyl-l-serine (ARA-S) mediates angiogenesis and wound healing through GPR-55 (Figure 1) [38]. CBD in a mouse model of dravet syndrome shows antagonism of GPR-55 effectively reduced seizures [39].
3. Phytocannabinoids and Their Use in Medicine
Phytocannabinoids are bioactive natural products found in some flowering plants, liverworts, and fungi and can be used to treat diseases or undesirable physiological symptoms [40]. Cannabis sativa is an abundant and most studied source of phytocannabinoids [40]. Currently, approximately 130 distinct phytocannabinoids have been isolated from cannabis plants and classified into several groups: cannabigerols (CBGs), cannabichromenes (CBCs), cannabidiols (CBDs), (−)-delta9-trans-tetrahydrocannabinols (delta9-THCs), (−)-delta8-trans-tetrahydrocannabinols (delta8-THCs), cannabicyclols (CBLs), cannabielsoins (CBEs), cannabinols (CBNs), cannabinodiols (CBNDs), cannabitriols (CBTs), and a few other miscellaneous phytocannabinoids [40]. The most abundant Cannabis sativa constituents are trans-delta 9-THC, CBD, CBC, and CBG, together with their respective acid forms (delta9-tetrahydrocannabinolic acid A (delta9-THCA), CBDA, CBCA, and CBGA) [40]. However, it is essential to note that phytocannabinoids and ECs mediate their effects as antagonists or agonists by interacting with the same specific cell-surface CBRs, essentially acting as ECs.
To date, the most utilized, popular, and intensively studied phytocannabinoids are delta9-THC and CBD, although lately, other phytocannabinoids derivatives, such as Δ9-tetrahydrocannabivarin (delta9-THCV), cannabinol (CBN), cannabidivarin (CBDV), cannabigerol (CBG), and cannabichromene (CBC), have become of significant interest [41]. Discovered in 1971, THC, the primary psychoactive compound of cannabis, was initially not widely used, but later led to the discovery of CBR-1 and a better definition of the ECS [42]. Today, THC is an approved medical therapy drug [43]. Nabiximols, a cannabis-based preparation containing a standardized ratio of THC and CBD, is an approved treatment for people with MS and has been used in the United Kingdom since 2010, then in Spain, Germany, Denmark, Sweden, Italy, Austria, and Poland since 2011–2012. France legalized cannabinoids in medicine in 2013 [44], and the brand name Sativex has been approved in Canada for cancer-related pain and was recently legalized in Ukraine. Moreover, THC products are commonly used as an effective remedy in a range of neurodegenerative and neurological disorders, such as Huntington, Parkinson’s, Alzheimer’s, and Tourette syndromes [45]. In addition, currently in the U.S., there are two chemically synthesized, cannabis-based drugs approved by the U.S. Food and Drug Administration (FDA) for use primarily in oncological patients. Dronabinol (Marinol) is a gelatin capsule containing an isomer of THC, used to relieve nausea and vomiting caused by chemotherapy or enhance the appetite to achieve weight gain in AIDS patients. Although it is a synthetic-based cannabinoid, Nabilone (Cesamet), acting much as THC, is another FDA-approved alternative drug to treat nausea and vomiting caused by chemotherapy [46].
CBD, the second most abundant phytocannabinoid, accounts for up to 40% of the plant’s extract and shows almost no affinity for CBR-1 and CBR-2, but it can antagonize the effect of THC and AEA to CBR-1 [47]. CBD does not have the same psychoactivity as THC and, thus far, CBD oil is mainly used to treat pain by affecting non-cannabinoid receptors, such as TRP channels and PPARs [47]. Although the U.S. removed hemp and hemp extracts (including CBD) from the list of controlled substances in 2018, and the FDA approved the first CBD-containing medical product, Epidiolex®, for the treatment of two rare epilepsy disorders, proving the tremendous medical potential of cannabis-based products [48], the marketing and sales of CBD formulations remain a grey area in many states. Contrary to the U.S., European Union member states allow CBD products legally sold as an antioxidant, anti-sebum, skin protection, and skincare (cosmetics) product, but they are restricted from being marketed as medical products improving health [48].
This entry is adapted from the peer-reviewed paper 10.3390/pharmaceutics14020389
References
- Cascio, M.G.; Marini, P. Biosynthesis and fate of endocannabinoids. Handb. Exp. Pharmacol. 2015, 231, 39–58.
- Hanuš, L.O. Discovery and isolation of anandamide and other endocannabinoids. Chem. Biodivers. 2007, 4, 1828–1841.
- Devane, W.A.; Hanuš, L.; Breuer, A.; Pertwee, R.G.; Stevenson, L.A.; Griffin, G.; Gibson, D.; Mandelbaum, A.; Etinger, A.; Mechoulam, R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 1992, 258, 1946–1949.
- Mechoulam, R.; Ben-Shabat, S.; Hanus, L.; Ligumsky, M.; Kaminski, N.E.; Schatz, A.R.; Gopher, A.; Almog, S.; Martin, B.R.; Compton, D.R.; et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem. Pharmacol. 1995, 50, 83–90.
- Gonsiorek, W.; Lunn, C.; Fan, X.; Narula, S.; Lundell, D.; Hipkin, R.W. Endocannabinoid 2-arachidonyl glycerol is a full agonist through human type 2 cannabinoid receptor: Antagonism by anandamide. Mol. Pharmacol. 2000, 57, 1045–1050.
- Duncan, M.; Millns, P.; Smart, D.; Wright, J.E.; Kendall, D.A.; Ralevic, V. Noladin ether, a putative endocannabinoid, attenuates sensory neurotransmission in the rat isolated mesenteric arterial bed via a non-CB1/CB2 G(i/o) linked receptor. Br. J. Pharmacol. 2004, 142, 509–518.
- Shoemaker, J.L.; Joseph, B.K.; Ruckle, M.B.; Mayeux, P.R.; Prather, P.L. The Endocannabinoid noladin ether acts as a full agonist at human CB2 cannabinoid receptors. J. Pharmacol. Exp. Ther. 2005, 314, 868–875.
- Porter, A.C.; Sauer, J.M.; Knierman, M.D.; Becker, G.W.; Berna, M.J.; Bao, J.; Nomikos, G.G.; Carter, P.; Bymaster, F.P.; Leese, A.B.; et al. Characterization of a novel endocannabinoid, virodhamine, with antagonist activity at the CB1 receptor. J. Pharmacol. Exp. Ther. 2002, 301, 1020–1024.
- Bisogno, T.; Ligresti, A.; Di Marzo, V. The endocannabinoid signalling system: Biochemical aspects. Pharmacol. Biochem. Behav. 2005, 81, 224–238.
- Howlett, A.C.; Barth, F.; Bonner, T.I.; Cabral, G.; Casellas, P.; Devane, W.A.; Felder, C.C.; Herkenham, M.; Mackie, K.; Martin, B.R.; et al. International union of pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol. Rev. 2002, 54, 161–202.
- Pertwee, R.G. Cannabinoid pharmacology: The first 66 years. Br. J. Pharmacol. 2006, 147, S163–S171.
- Nyíri, G.; Cserép, C.; Szabadits, E.; Mackie, K.; Freund, T.F. CB1 cannabinoid receptors are enriched in the perisynaptic annulus and on preterminal segments of hippocampal GABAergic axons. Neuroscience 2005, 136, 811–822.
- Svíženská, I.; Dubový, P.; Šulcová, A. Cannabinoid Receptors 1 and 2 (CB1 and CB2), Their distribution, ligands and functional involvement in nervous system structures—A short review. Pharmacol. Biochem. Behav. 2008, 90, 501–511.
- Galiègue, S.; Mary, S.; Marchand, J.; Dussossoy, D.; Carrière, D.; Carayon, P.; Bouaboula, M.; Shire, D.; le Fur, G.; Casellas, P. Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur. J. Biochem. 1995, 232, 54–61.
- Pertwee, R.G. Pharmacology of cannabinoid CB1 and CB2 receptors. Pharmacol. Ther. 1997, 74, 129–180.
- Spigelman, I. Therapeutic targeting of peripheral cannabinoid receptors in inflammatory and neuropathic pain states. In Translational Pain Research: From Mouse to Man; CRC Press: Boca Raton, FL, USA, 2010; pp. 99–138.
- Pertwee, R.G.; Ross, R.A. Cannabinoid receptors and their ligands. Prostaglandins Leukot. Essent. Fat. Acids 2002, 66, 101–121.
- Munro, S.; Thomas, K.L.; Abu-Shaar, M. Molecular characterization of a peripheral receptor for cannabinoids. Nature 1993, 365, 61–65.
- Nong, L.; Newton, C.; Cheng, Q.; Friedman, H.; Roth, M.D.; Klein, T.W. Altered cannabinoid receptor mRNA expression in peripheral blood mononuclear cells from marijuana smokers. J. Neuroimmunol. 2002, 127, 169–176.
- Gardner, B.; Zu, L.X.; Sharma, S.; Dubinett, S.M.; Gardner, B.; Zu, L.X.; Sharma, S.; Tashkin, D.P.; Dubinett, S.M.; Sharma, S.; et al. Autocrine and paracrine regulation of lymphocyte CB2 receptor expression by TGF-beta. Biochem. Biophys. Res. Commun. 2002, 290, 91–96.
- Racz, I.; Nadal, X.; Alferink, J.; Baños, J.E.; Rehnelt, J.; Martín, M.; Pintado, B.; Gutierrez-Adan, A.; Sanguino, E.; Bellora, N.; et al. Interferon-γ is a critical modulator of CB2 cannabinoid receptor signaling during neuropathic pain. J. Neurosci. 2008, 28, 12136.
- Maresz, K.; Carrier, E.J.; Ponomarev, E.D.; Hillard, C.J.; Dittel, B.N. Modulation of the cannabinoid CB2 receptor in microglial cells in response to inflammatory stimuli. J. Neurochem. 2005, 95, 437–445.
- Duncan, M.; Galic, M.A.; Wang, A.; Chambers, A.P.; McCafferty, D.M.; McKay, D.M.; Sharkey, K.A.; Pittman, Q.J. Cannabinoid 1 receptors are critical for the innate immune response to TLR4 stimulation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 305, R224–R231.
- Gui, H.; Sun, Y.; Luo, Z.M.; Su, D.F.; Dai, S.M.; Liu, X. Cannabinoid receptor 2 protects against acute experimental sepsis in mice. Media. Inflamm. 2013, 2013, 741303.
- O’Sullivan, S.E. An update on PPAR activation by cannabinoids. Br. J. Pharmacol. 2016, 173, 1899.
- O’sullivan, S.E. Cannabinoids go nuclear: Evidence for activation of peroxisome proliferator-activated receptors. Br. J. Pharmacol. 2007, 152, 576–582.
- Iannotti, F.A.; Vitale, R.M. The endocannabinoid system and PPARs: Focus on their signalling crosstalk, action and transcriptional regulation. Cells 2021, 10, 586.
- Muller, C.; Morales, P.; Reggio, P.H. Cannabinoid ligands targeting TRP channels. Front. Mol. Neurosci. 2019, 11, 487.
- Khalil, M.; Alliger, K.; Weidinger, C.; Yerinde, C.; Wirtz, S.; Becker, C.; Engel, M.A. Functional Role of transient receptor potential channels in immune cells and epithelia. Front. Immunol. 2018, 9, 174.
- Zygmunt, P.M.; Petersson, J.; Andersson, D.A.; Chuang, H.H.; Sørgård, M.; Di Marzo, V.; Julius, D.; Högestätt, E.D. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature 1999, 400, 452–457.
- De Petrocellis, L.; Starowicz, K.; Moriello, A.S.; Vivese, M.; Orlando, P.; Di Marzo, V. Regulation of transient receptor potential channels of melastatin type 8 (TRPM8): Effect of cAMP, cannabinoid CB(1) receptors and endovanilloids. Exp. Cell Res. 2007, 313, 1911–1920.
- De Petrocellis, L.; Ligresti, A.; Moriello, A.S.; Allarà, M.; Bisogno, T.; Petrosino, S.; Stott, C.G.; Di Marzo, V. Effects of cannabinoids and cannabinoid-enriched cannabis extracts on TRP channels and endocannabinoid metabolic enzymes. Br. J. Pharmacol. 2011, 163, 1479–1494.
- Yang, D.; Zhou, Q.; Labroska, V.; Qin, S.; Darbalaei, S.; Wu, Y.; Yuliantie, E.; Xie, L.; Tao, H.; Cheng, J.; et al. G Protein-coupled receptors: Structure- and function-based drug discovery. Signal Transduct. Target. Ther. 2021, 6, 7.
- Wacker, D.; Stevens, R.C.; Roth, B.L. How ligands illuminate GPCR molecular pharmacology. Cell 2017, 170, 414.
- Wang, D. The essential role of G protein-coupled receptor (GPCR) signaling in regulating T cell immunity. Immunopharmacol. Immunotoxicol. 2018, 40, 187–192.
- Pacher, P.; Kogan, N.M.; Mechoulam, R. Beyond THC and endocannabinoids. Annu. Rev. Pharmacol. Toxicol. 2020, 60, 637–659.
- Morales, P.; Lago-Fernandez, A.; Hurst, D.P.; Sotudeh, N.; Brailoiu, E.; Reggio, P.H.; Abood, M.E.; Jagerovic, N. Therapeutic exploitation of GPR18: Beyond the cannabinoids? J. Med. Chem. 2020, 63, 14216–14227.
- Zhang, X.; Maor, Y.; Wang, J.F.; Kunos, G.; Groopman, J.E. Endocannabinoid-like N-arachidonoyl serine is a novel pro-angiogenic mediator. Br. J. Pharmacol. 2010, 160, 1583.
- Kaplan, J.S.; Stella, N.; Catterall, W.A.; Westenbroek, R.E. Cannabidiol attenuates seizures and social deficits in a mouse model of dravet syndrome. Proc. Natl. Acad. Sci. USA 2017, 114, 11229–11234.
- Gülck, T.; Møller, B.L. Phytocannabinoids: Origins and biosynthesis. Trends Plant Sci. 2020, 25, 985–1004.
- Gaoni, Y.; Mechoulam, R.; Gaoni, Y.; Mechoulam, R. Isolation and structure of.DELTA.+- tetrahydrocannabinol and other neutral cannabinoids from hashish. J. Am. Chem. Soc. 2002, 93, 217–224.
- Matsuda, L.A.; Lolait, S.J.; Brownstein, M.J.; Young, A.C.; Bonner, T.I. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 1990, 346, 561–564.
- Bridgeman, M.B.; Abazia, D.T. Medicinal cannabis: History, pharmacology, and implications for the acute care setting. Pharm. Ther. 2017, 42, 180.
- Bramness, J.G.; Dom, G.; Gual, A.; Mann, K.; Wurst, F.M. A survey on the medical use of cannabis in Europe: A position paper. Eur. Addict. Res. 2018, 24, 201–205.
- Koppel, B.S.; Brust, J.C.M.; Fife, T.; Bronstein, J.; Youssof, S.; Gronseth, G.; Gloss, D. Systematic review: Efficacy and safety of medical marijuana in selected neurologic disorders: Report of the guideline development subcommittee of the American academy of neurology. Neurology 2014, 82, 1556–1563.
- Abu-Amna, M.; Salti, T.; Khoury, M.; Cohen, I.; Bar-Sela, G. Medical cannabis in oncology: A valuable unappreciated remedy or an undesirable risk? Curr. Treat. Options Oncol. 2021, 22, 16.
- Vučkovic, S.; Srebro, D.; Vujovic, K.S.; Vučetic, Č.; Prostran, M. Cannabinoids and pain: New insights from old molecules. Front. Pharmacol. 2018, 9, 1259.
- Vlad, R.A.; Hancu, G.; Ciurba, A.; Antonoaea, P.; Rédai, E.M.; Todoran, N.; Silasi, O.; Muntean, D.L. Cannabidiol-therapeutic and legal aspects. Pharmazie 2020, 75, 463–469.
This entry is offline, you can click here to edit this entry!
