Diels–Alder cycloaddition reaction is one of the most powerful strategies for the construction of six-membered carbocyclic and heterocyclic systems, in most cases with high regio- and stereoselectivity. In this review, an insight into the most relevant advances on sustainable Diels–Alder reactions since 2010 is provided. Various environmentally benign solvent systems are discussed, namely bio-based derived solvents, polyethylene glycol, deep eutectic solvents, supercritical carbon dioxide, water and water-based aqueous systems.
- Diels–Alder reaction
- green solvent
- bio-based solvent
- deep eutectic solvents
- water
- green chemistry
1. Introduction
2. Bio-based solvents
Glycerol, the main by-product in the biodiesel industry, is a nontoxic, biodegradable, recyclable and inexpensive viscous liquid. These properties, allied with the high stability, biocompatibility and ability to dissolve organic compounds poorly miscible in water as well as inorganic compounds, make glycerol a valuable green solvent in synthetic organic chemistry [38][39][40].
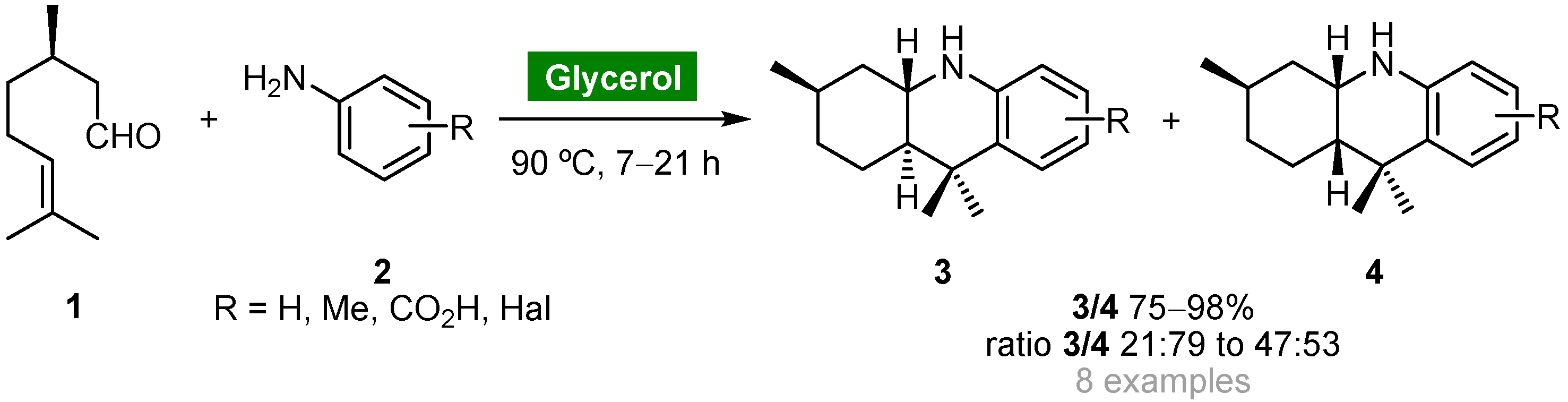
The three-component aza-Diels–Alder reaction of substituted anilines, aldehydes and electron-rich alkenes, also known as three-component imino-Diels–Alder reaction, or multicomponent Povarov reaction, is one of the most straightforward, efficient and atom-economical strategies towards complex cores starting from simple, inexpensive and available materials. Perin and coworkers explored the intramolecular version of this reaction for the catalyst-free synthesis of octahydroacridines starting from (R)-citronellal (1) and substituted arylamines 2 using glycerol as a recyclable and eco-friendly solvent (Scheme 1) [41]. Cycloadducts 3 and 4 were obtained as diastereoisomeric mixtures in good to high yields (75–98%) and moderate cis-selectivity when the reaction was carried out at 90 °C. Cycloadducts 3/4 (R = H) were obtained in lower yield (62%) using water as solvent, whereas the reaction carried out in organic solvents afforded the corresponding adducts in only trace amounts. Due to the insolubility of 3 and 4 in glycerol, products could be removed from the reaction medium by decantation, and the solvent could be reused for further aza-Diels–Alder reactions without loss of activity.
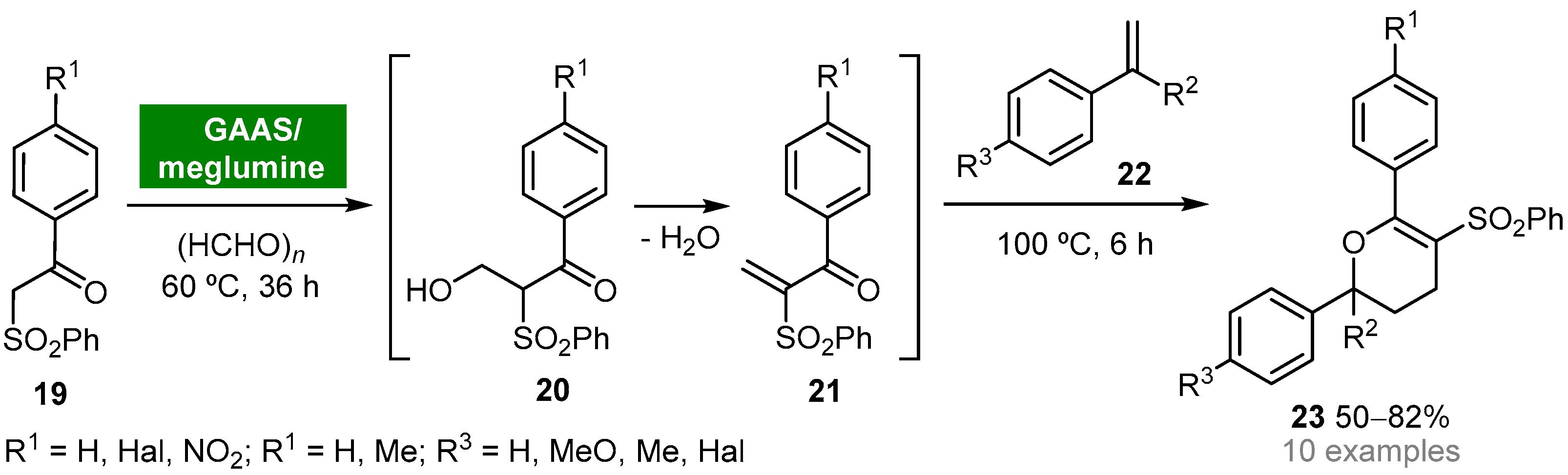
Gluconic acid (GA) can be obtained from biomass and possesses the ideal properties for being classified as a green and sustainable solvent (e.g., nontoxicity, biodegradability, recyclability, high boiling point, low vapor pressure) [42]. Due to the high solubility of gluconic acid in water, gluconic acid aqueous solutions (GAASs) have found wide application as solvent media for organic reactions, namely for the Knoevenagel condensation reaction. The Gu group reported the synthesis of 2H-pyrans by a one-pot multicomponent reaction between β-ketosulfones, formaldehyde and styrenes in a bio-based binary mixture solvent system composed of GAAS and a sugar-based organic base, meglumine [43]. The disclosed protocol involves the in situ generation of α-methylene-β-ketosulfones 21 through a Knoevenagel reaction of β-ketosulfones 19 and formaldehyde. Next, nucleophilic trap of 21 with styrenes 22 via oxa-Diels–Alder reaction afforded 2,6-diaryl-5-(phenylsulfonyl)-3,4-dihydro-2H-pyrans 23 in moderate to good yields (50–82%) (Scheme 2). The binary solvent system GAAS/meglumine proved to play a pivotal role in controlling the selectivity of the hydroxymethylation step. Moreover, the hydrophilic properties of bio-based solvent meglumine allowed it to be easily recycled and reused in the GAAS/meglumine system without significant loss of activity.
3. Polyethylene Glycol
Polyethylene glycol (PEG), HO–(CH2CH2O)n–H, is a biodegradable, nontoxic, odorless, neutral, nonvolatile and inexpensive water-soluble polymer that has found widespread application as a green reaction medium for several organic transformations [20]. The Kouznetsov group reported the diastereoselective synthesis of heterolignan-like 6,7-methylendioxy-tetrahydroquinolines via a BF3.OEt-catalyzed three-component Povarov reaction using clove bud essential oil as a renewable raw material and PEG-400 as green solvent (Scheme 3) [44]. Clove bud essential oil enriched with eugenol 24 (60.5%) was obtained by hydrodistillation of dried flower buds and then subjected to a solid base-catalyzed isomerization to give trans/cis-isoeugenol 25, which could be used as a dienophile in the multicomponent hetero-Diels–Alder reaction without further purification. The reaction of 25 with aldimines generated in situ from substituted benzaldehydes 27 and 3,4-(methylendioxy)aniline (26) afforded trans-2,4-diaryl-1,2,3,4-tetrahydroquinolines 28 as racemic mixtures in moderate yields (35–55%). The reaction with phthalaldehydic acid (29) afforded isoindolo[2,1-a]quinolin-11(5H)-one 30 via an intramolecular condensation of the initially generated NH-tetrahydroquinoline core with the o-carboxylic acid function leading to the formation of the γ-lactam ring. It is noteworthy that these reactions also worked using acetonitrile as solvent media; however, less solvent volume and reduced reaction times were required when using PEG-400.
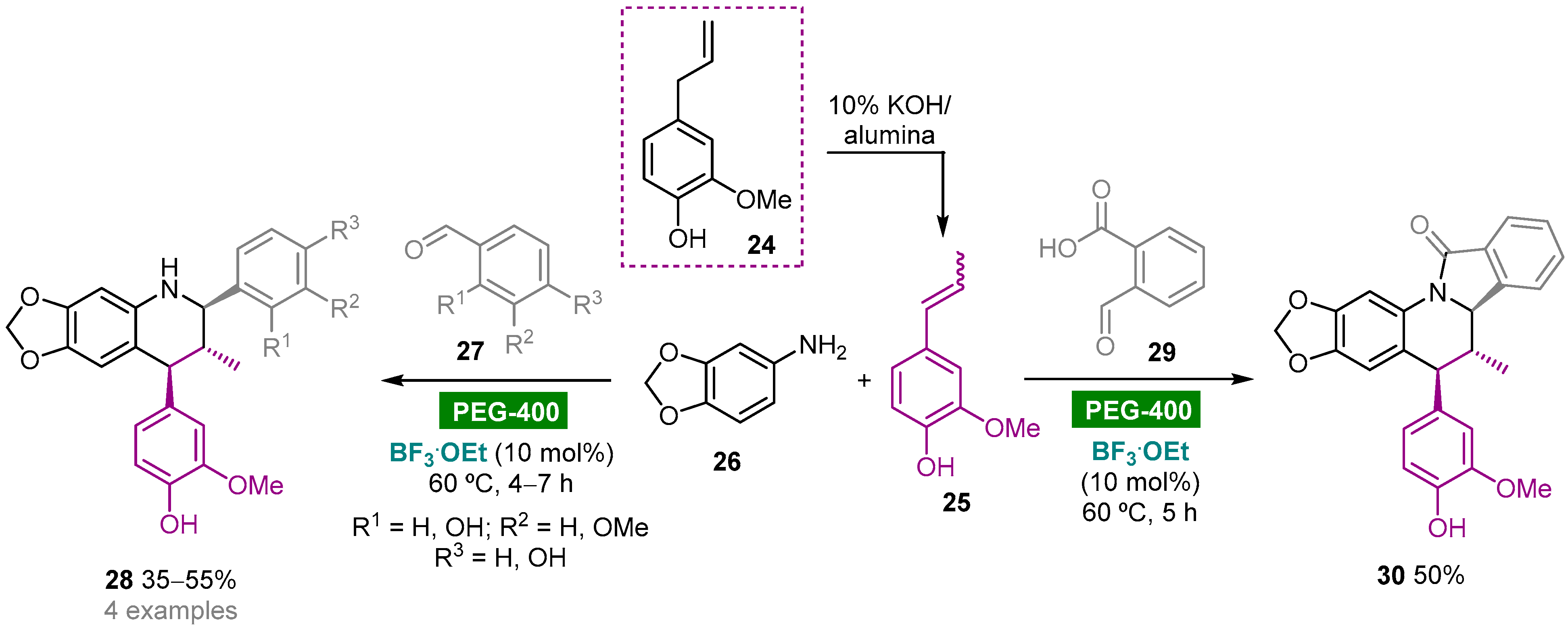
Scheme 3. BF3.OEt-catalyzed one-pot multicomponent aza-Diels–Alder reaction of 3,4-(methylendioxy)aniline, aromatic aldehydes and trans/cis-isoeugenol in PEG-400.
4. Organic Carbonates
Propylene carbonate (PC) is a polar aprotic solvent that can be obtained from propylene oxide and carbon dioxide, a renewable source of carbon, in a 100% atom economy reaction with relevance regarding the development of CO2 fixation processes. The noncorrosive, nontoxic, odorless and biodegradable properties of PC, allied with high boiling point, low vapor pressure and low cost, make this solvent a green and sustainable alternative to conventional organic solvents [45]. The Povarov reaction has also been explored using PC as an environmentally friendly solvent [46]. The one-pot iodine-catalyzed reaction of mono- or disubstituted anilines 2, aromatic aldehydes 5 and isoeugenol (45) carried out at room temperature using PC as solvent medium afforded functionalized tetrahydroquinolines 46 in good to high yields (77–95%) and high diastereoselectivity (dr up to >99:1) (Scheme 4). The same cycloadducts were obtained using organic solvents, albeit in low yields and requiring longer reaction times. It is noteworthy that, in general, products precipitated from the reaction medium and were purified by recrystallization.
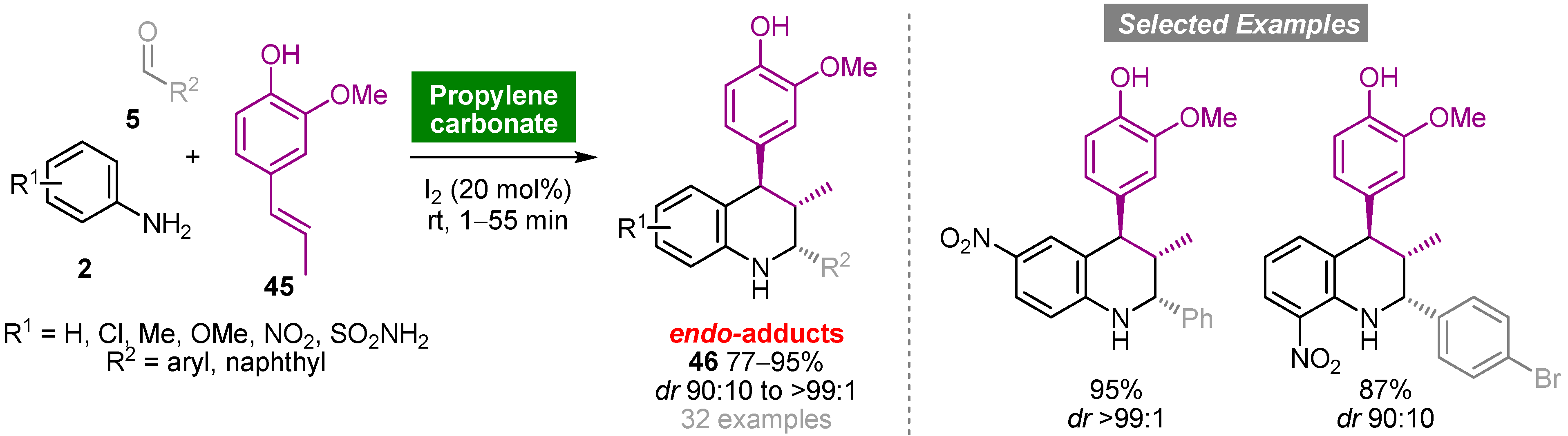
Scheme 4. Iodine-catalyzed one-pot multicomponent aza-Diels–Alder reaction of anilines, aromatic aldehydes and isoeugenol in propylene carbonate.
5. Deep Eutectic Solvents
First introduced by Abbot [47], deep eutectic solvents (DESs) are low melting mixtures obtained by combination of at least two components, a hydrogen bond acceptor (HBA), generally a quaternary ammonium or metal salt, and a hydrogen bond donor (HBD), to form a eutectic phase via hydrogen bond interactions. DESs are characterized by a melting point lower than those of the single components. The properties of DESs are very similar to those of room-temperature ionic liquids; however, the main difference from ionic liquids is that DESs also contain an organic molecular component, the HBD (e.g., urea, amide, polyol), generally as a major component. Due to their low vapor pressure, nonflammability, thermal and chemical stability, nontoxicity, biodegradability, recyclability and low price, DESs have emerged as green and sustainable media in different areas of chemical research [48][49][50][51], namely organic synthesis and catalysis [23][24][25][26].
Garcia-Álvarez’s group reported a one-pot tandem cycloisomerization/Diels–Alder reaction using a ChCl (choline chloride)-based eutectic mixture as solvent [52]. The protocol involves the in situ generation of furans 59 by cycloisomerization of (Z)-enynols 57 using a ChCl/Gly (1:2) eutectic mixture as solvent and bis(iminophosphorane)-Au(i) complex 58 as catalyst (Scheme 5). The Diels–Alder reaction of furans 59 with activated alkynes 60 afforded 7-oxanorbornadienes 61, whereas the reaction with activated alkenes, 55b or 62, afforded selectively exo-7-oxanorbornenes 63. The authors have demonstrated that complex 58 is crucial for the cycloisomerization step; however, it does not participate in the cycloaddition step.
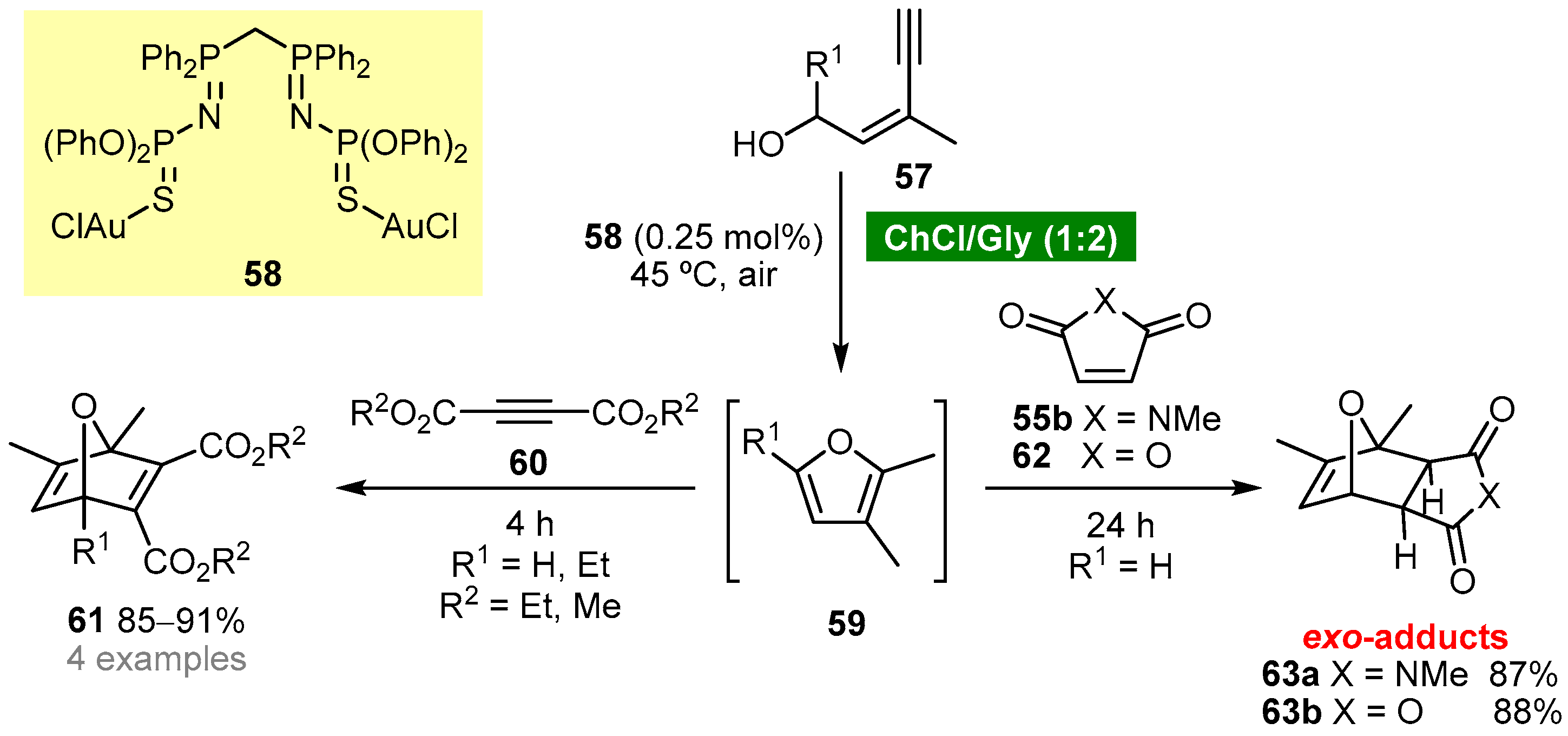
Scheme 5. One-pot tandem cycloisomerization/Diels–Alder reaction of (Z)-enynols in a ChCl/Gly deep eutectic solvent.
6. Supercritical Carbon Dioxide
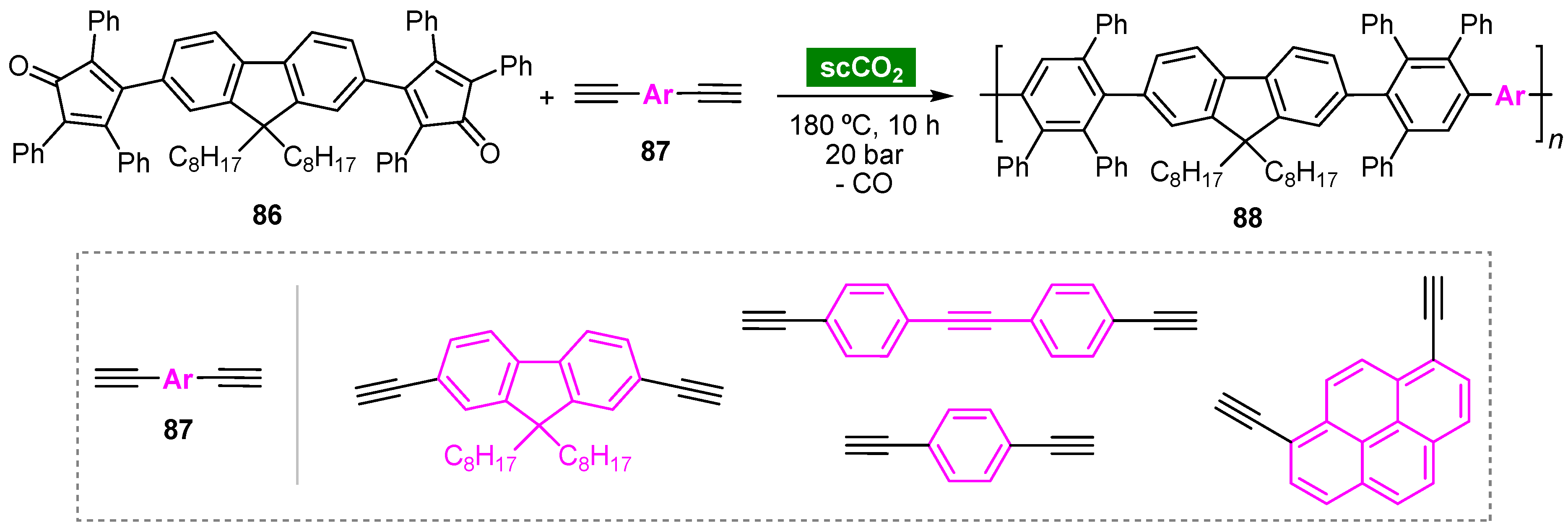

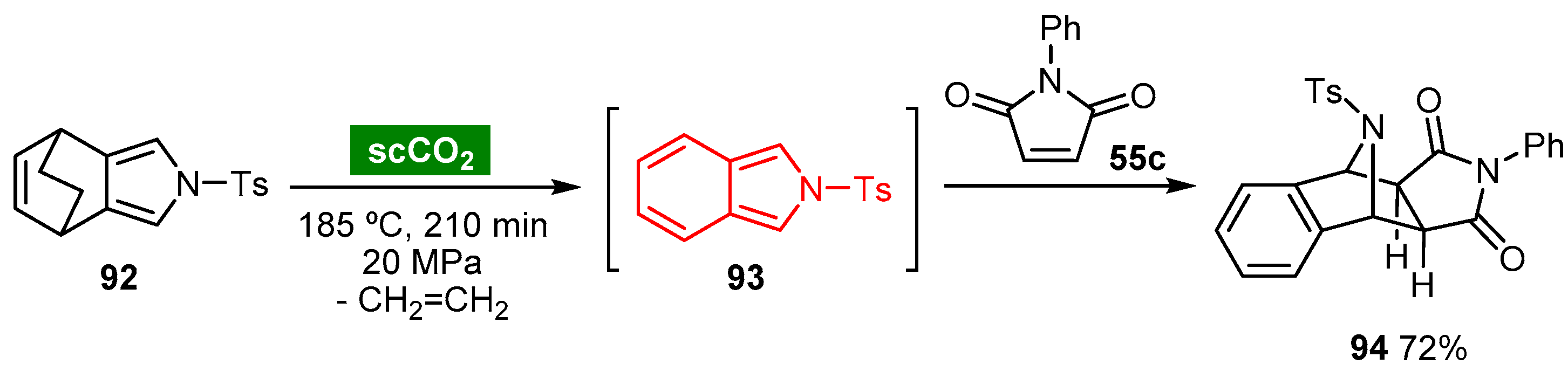
This entry is adapted from the peer-reviewed paper 10.3390/molecules27041304
References
- Díaz-Álvarez, A.E.; Francos, J.; Lastra-Barreira, B.; Crochet, P.; Cadierno, V. Glycerol and derived solvents: New sustainable reaction media for organic synthesis. Chem. Commun. 2011, 47, 6208–6227.
- Díaz-Álvarez, A.E.; Francos, J.; Crochet, P.; Cadierno, V. Recent advances in the use of glycerol as green solvent for synthetic organic chemistry. Curr. Green Chem. 2014, 1, 51–65.
- Abd-Elmonem, M.; Mekheimer, R.A.; Hayallah, A.M.; Abo-Elsoud, F.; Sadek, K.U. Recent advances in the utility of glycerol as a benign and biodegradable medium in heterocyclic synthesis. Curr. Org. Chem. 2019, 23, 3226–3246.
- Nascimento, J.E.R.; Barcellos, A.M.; Sachini, M.; Perin, G.; Lenardão, E.J.; Alves, D.; Jacob, R.G.; Missau, F. Catalyst-free synthesis of octahydroacridines using glycerol as recyclable solvent. Tetrahedron Lett. 2011, 52, 2571–2574.
- Lim, H.Y.; Dolzhenko, A.V. Gluconic acid aqueous solution: A bio-based catalytic medium for organic synthesis. Sustain. Chem. Pharm. 2021, 21, 100443.
- Yang, J.; Li, H.; Li, M.; Peng, J.; Gu, Y. Multicomponent reactions of β-ketosulfones and formaldeyde in a bio-based binary mixture solvent system composed of meglumine and gluconic acid aqueous solution. Adv. Synth. Catal. 2012, 354, 688–700.
- Chen, J.; Spear, S.K.; Huddleston, J.G.; Rogers, R.D. Polyethylene glycol and solutions of polyethylene glycol as green reaction media. Green Chem. 2005, 7, 64–82.
- Arenas, D.R.M.; Ruíz, F.A.R.; Kouznetsov, V.V. Highly diastereoselective synthesis of new heterolignan-like 6,7-methylendioxy-tetrahydroquinolines using the clove bud essential oil as raw material. Tetrahedron Lett. 2011, 52, 1388–1391.
- Forero, J.S.B.; Muñoz, J.A.H.; Jones, J.; da Silva, F.M. Propylene carbonate in organic synthesis: Exploring its potential as a green solvent. Curr. Org. Synth. 2016, 13, 834–846.
- Forero, J.S.B.; de Carvalho, E.M.; Junior, J.J.; da Silva, F.M. Facile, efficient diastereoselective synthesis of tetrahydroquinoline scaffolds using propylene carbonate as an eco-friendly solvent. Curr. Org. Synth. 2015, 12, 102–107.
- Abbott, A.P.; Boothby, D.; Capper, G.; Davies, D.L.; Rasheed, R.K. Deep eutectic solvents formed between choline chloride and carboxylic acids: Versatile alternatives to ionic liquids. J. Am. Chem. Soc. 2004, 126, 9142–9147.
- Zhang, Q.; Vigier, K.O.; Royer, S.; Jérôme, F. Deep eutectic solvents: Syntheses, properties and applications. Chem. Soc. Rev. 2012, 41, 7108–7146.
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep eutectic solvents (DESs) and their applications. Chem. Rev. 2014, 114, 11060–11082.
- Yu, D.; Xue, Z.; Mu, T. Eutectics: Formation, properties, and applications. Chem. Soc. Rev. 2021, 50, 8596–8638.
- Hansen, B.B.; Spittle, S.; Chen, B.; Poe, D.; Zhang, Y.; Klein, J.M.; Horton, A.; Adhikari, L.; Zelovich, T.; Doherty, B.W.; et al. Deep eutectic solvents: A review of fundamentals and applications. Chem. Rev. 2021, 121, 1232–1285.
- Wang, A.; Zheng, X.; Zhao, Z.; Li, C.; Zheng, X. Deep eutectic solvents to organic synthesis. Prog. Chem. 2014, 26, 784–795.
- Liu, P.; Hao, J.-W.; Mo, L.-P.; Zhang, Z.-H. Recent advances in the application of deep eutectic solvents as sustainable media as well as catalysts in organic reactions. RSC Adv. 2015, 5, 48675–48704.
- Khandelwal, S.; Tailor, Y.K.; Kumar, M. Deep eutectic solvents (DESs) as eco-friendly and sustainable solvent/catalyst systems in organic transformations. J. Mol. Liq. 2016, 215, 345–386.
- Alonso, D.A.; Baeza, A.; Chinchilla, R.; Guillena, G.; Pastor, I.M.; Ramón, D.J. Deep eutectic solvents: The organic reaction medium of the century. Eur. J. Org. Chem. 2016, 2016, 612–632.
- Vidal, C.; Merz, L.; García-Álvarez, J. Deep eutectic solvents: Biorenewable reaction media for Au(I)-catalysed cycloisomerisations and one-pot tandem cycloisomerisation/Diels–Alder reactions. Green Chem. 2015, 17, 3870–3878.
- Eckert, C.A.; Knutson, B.L.; Debenedetti, P.G. Supercritical fluids as solvents for chemical and materials processing. Nature 1996, 383, 313–318.
- Peach, J.; Eastoe, J. Supercritical carbon dioxide: A solvent like no other. Beilstein J. Org. Chem. 2014, 10, 1878–1895.
- Kaupp, G. Reactions in supercritical carbon dioxide. Angew. Chem. Int. Ed. 1994, 33, 1452–1455.
- Oakes, R.S.; Clifford, A.A.; Rayner, C.M. The use of supercritical fluids in synthetic organic chemistry. J. Chem. Soc. Perkin Trans. 2001, 1, 917–941.
- Licence, P.; Ke, J.; Sokolova, M.; Ross, S.K.; Poliakoff, M. Chemical reactions in supercritical carbon dioxide: From laboratory to commercial plant. Green Chem. 2003, 5, 99–104.
- Beckman, E.J. Supercritical and near-critical CO2 in green chemical synthesis and processing. J. Supercrit. Fluids 2004, 28, 121–191.
- Savage, P.E.; Gopalan, S.; Mizan, T.I.; Martino, C.J.; Brock, E.E. Reactions at supercritical conditions: Applications and fundamentals. AIChE J. 1995, 41, 1723–1778.
- Keshtov, M.L.; Mal’tsev, E.I.; Lopatin, A.M.; Nikitin, L.N.; Marochkin, D.V.; Perevalov, V.P.; Petrovskii, P.V.; Khokhlov, A.R. Photoluminescent phenylated polyfluorenes synthesized in an organic solvent and supercritical carbon dioxide. Polym. Sci. Ser. B 2012, 54, 106–114.
- Meng, F.-Q.; Feng, X.-J.; Wang, W.-H.; Bao, M. Synthesis of 5-vinyl-2-norbornene through Diels–Alder reaction of cyclopentadiene with 1,3-butadiene in supercritical carbon dioxide. Chin. Chem. Lett. 2017, 28, 900–904.
- Ito, S.; Akaki, M.; Shinozaki, Y.; Iwabe, Y.; Furuya, M.; Tobata, M.; Roppongi, M.; Sato, T.; Itoh, N.; Oba, T. Efficient synthesis of isoindoles using supercritical carbon dioxide. Tetrahedron Lett. 2017, 58, 1338–1342.
- Savage, P.E.; Gopalan, S.; Mizan, T.I.; Martino, C.J.; Brock, E.E. Reactions at supercritical conditions: Applications and fundamentals. AIChE J. 1995, 41, 1723–1778.
- Hailes, H.C. Reaction solvent selection: The potential of water as a solvent for organic transformations. Org. Process Res. Dev. 2007, 11, 114–120.
- Chanda, A.; Fokin, V.V. Organic synthesis “on water”. Chem. Rev. 2009, 109, 725–748.
- Gawande, M.B.; Bonifácio, V.D.B.; Luque, R.; Branco, P.S.; Varma, R.S. Benign by design: Catalyst-free in-water, on-water green chemical methodologies in organic synthesis. Chem. Soc. Rev. 2013, 42, 5522–5551.
- Butler, R.N.; Coyne, A.G. Organic synthesis reactions on-water at the organic-liquid water interface. Org. Biomol. Chem. 2016, 14, 9945–9960.
- Kitanosono, T.; Masuda, K.; Xu, P.Y.; Kobayashi, S. Catalytic organic reactions in water toward sustainable society. Chem. Rev. 2018, 118, 679–746.
- Bhowmick, K.C.; Bihani, M.; Zhao, J.C.-G. Organocatalyzed asymmetric Diels–Alder reactions in aqueous or semi-aqueous media. Mini Rev. Org. Chem. 2018, 15, 3–19.
