Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Plant Sciences
The frequency and severity of extreme climatic conditions such as drought, salinity, cold, and heat are increasing due to climate change. Moreover, in the field, plants are affected by multiple abiotic stresses simultaneously or sequentially. Peanut metabolomics identified 160 metabolites, including amino acids, sugars, sugar alcohols, organic acids, fatty acids, sugar acids, and other organic compounds. Pathway enrichment analysis revealed that abiotic stresses significantly affected amino acid, amino sugar, and sugar metabolism.
- heat
- metabolomics
- peanut
1. Introduction
The frequency and severity of abiotic stresses are increasing due to climate change and global warming [1]. Researchers generally study a single stressor to evaluate the mechanism or effect on plants. However, multiple abiotic and biotic stresses can simultaneously affect plants under field conditions [2]. Standard laboratory conditions that are often used for plant science research significantly differ from the field, so it is difficult to associate output results from an individual stress study to field conditions. Abiotic stresses can lead to the production of excess reactive oxygen species (ROS), mainly in chloroplasts, mitochondria, and peroxisomes, with detrimental effects on signaling behavior [3][4]. Compared to individual stresses, combined abiotic stresses respond differently to ROS production through the differential production of enzymatic and non-enzymatic antioxidants in plant cells, resulting in a unique ROS signature and acclimation response via modifications to the signaling pathway [2][5][6]. Plants under combined abiotic stresses also differ from those that are under individual stresses for photosynthesis, stomatal regulation, and water use efficiency (WUE) [7][8]. For example, the net photosynthesis rate of soybean decreased more under combined water deficit and heat stress than individual stresses due to reduced CO2 availability, lower relative water content (RWC), and higher leaf temperature [9][10]. Similarly, WUE (directly linked to stomatal opening or closing) decreased in most studies under different stress combinations [11][12]. Combined abiotic stresses significantly reduce crop productivity and yield by affecting plant reproductive processes [13]. Recent studies in maize and wheat showed that combined abiotic stresses considerably decreased crop yield by reducing stigma functionality and kernel abortion [14][15].
Metabolomics is an emerging technology in plant biology representing data output from gene expression, protein interaction, and pathway regulations. Untargeted metabolic profiling of plant samples under different abiotic stresses is a new dimension for plant metabolic pathway and signaling research [16]. Metabolomics of rice flowering organs under combined drought and heat stress revealed that ribitol, pyruvic acid, and succinic acid significantly correlated with yield and the chalky grain fraction of seeds. In addition, the combined stress significantly increased the arbutin levels in flag leaves; this glycoside has strong antioxidant and membrane-stabilizing properties [17]. Eucalyptus under combined heat and drought stress significantly decreased WUE and differential metabolite accumulation compared to individual stresses [18]. Organic acids and carbohydrates, such as succinate, malate, quinate, glycerate, mannose, and galactose, significantly decreased, while most amino acids, including aspartate, glutamate, aspargine, valine, leucine, isoleucine, proline threonine, lysine, and histidine, significantly increased under combined stress [18]. Metabolomics can also be used to identify quantitative trait loci (QTL) and markers under various stresses, such as those that are related to the antioxidant enzyme system that was identified in barley and potato under combined drought and heat stress [19][20].
Peanut or groundnut (Arachis hypogaea L.) is a major oilseed legume that is grown in subtropical and tropical regions [21]. Peanut is a rich source of oil (40–60%), protein (10–20%), carbohydrates, vitamins, minerals, antioxidants, and monounsaturated fatty acids, and a source of medicinally important compounds [22][23]. India is the world’s largest edible oil consumer and peanut oil is the third-most consumed edible oil in India after palm and soybean oil. Abiotic stresses such as drought, salinity, and heat frequently affect peanut production, as it mainly grows in subtropical and tropical regions. Therefore, efforts are underway to improve abiotic stress tolerance in peanut [21][24][25][26]. Some studies have investigated the effect of abiotic stresses on physiological, biochemical, and metabolic changes in peanut [22][23][27][28]. One study evaluated the physiological and biochemical characteristics of ancestral peanut species under drought stress, revealing Arachis ipaensis as the most drought-tolerant due to higher solute accumulation in the roots than the other varieties [22]. A metabolomics study reported that drought-tolerant peanut varieties accumulated important polyamines and polyphenols such as agmatine, cadaverine, syringic acid, and vanillic acid under stress [23].
2. Biochemical Status of Plants under Different Stress Conditions
In peanut, the various stresses adversely affected the biochemical constituents, including sugars, starch, amino acids, and polyphenols (Figure 1). Free amino acids (FAA) increased in all the stress treatments except for individual cold stress and cold-containing combined stresses, relative to the control (unstressed) plants (Figure 1a). The maximum increases in FAA occurred under D-H (11.64 ± 0.31 mg g−1, 9.65-fold) and S-H (11.10 ± 0.73 mg g−1, 9.2-fold) stress followed by S-D-H (8.06 ± 0.23 mg g−1, 6.68-fold) and heat (5.33 ± 0.85 mg g−1, 4.4-fold) stress, compared to the unstressed plants (1.20 ± 0.20 mg g−1). In contrast, the smallest increments in FAA occurred in plants that were grown under combined stress that included cold [S-C (2.12 ± 0.15 mg g−1, 1.76-fold), D-C (2.05 ± 0.11 mg g−1, 1.70-fold), and S-D-C (2.38 ± 0.07 mg g−1, 1.97-fold)].
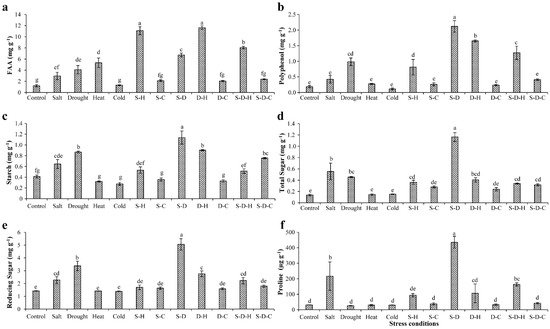
Figure 1. Biochemical status of plants under different stress conditions. Estimation of (a) free amino acids, (b) polyphenols, (c) starch, (d) total sugars, (e) reducing sugars, and (f) proline in peanut under individual and combined stresses (S-D: salinity and drought, S-H: salinity and heat, S-C: salinity and cold, D-H: drought and heat, D-C: drought and cold, S-D-H: salinity, drought, and heat, and S-D-C: salinity, drought, and cold). The data are the mean ± SE; different letters indicate significant differences at p < 0.05.
The polyphenol contents significantly increased in individual drought stress (0.98 ± 0.12 mg g−1, 5.32-fold) and S-D (2.12 ± 0.18 mg g−1, 11.45-fold), D-H (1.65 ± 0.03 mg g−1, 8.92-fold), S-H (0.81 ± 0.24 mg g−1, 4.39-fold), and S-D-H (1.27 ± 0.21 mg g−1, 6.87-fold) stresses compared to the control plants (Figure 1b). Similarly, the starch content significantly increased under individual drought stress (0.86 ± 0.01 mg g−1, 2.1-fold) and combined stress that included drought [S-D (1.13 ± 0.12 mg g−1, 2.75-fold), D-H (0.90 ± 0.01 mg g−1, 2.18-fold), S-D-C (0.75 ± 0.01 mg g−1, 1.83-fold)] compared to the unstressed plants (0.41 ± 0.02 mg g−1) (Figure 1c). A similar pattern occurred for the total and reducing sugar concentrations (Figure 1d,e). The sugar contents significantly increased under individual drought stress (total sugars, 1.16 ± 0.07 mg g−1, 8.37-fold; reducing sugars, 5.06 ± 0.44 mg g−1, 3.56-fold) compared to the control plants (total sugars, 0.13 ± 0.01 mg g−1; reducing sugars, 1.42 ± 0.01 mg g−1).
Proline provides abiotic stress tolerance to plants by modulating osmotic adjustment [29]. The elevated proline content occurred in plants that were grown under individual salt stress and combined stresses that included salt (Figure 1f), more so for S-D (435.20 ± 37.70 µg g−1, 14.16-fold) compared to the control (30.72 ± 0.47 µg g−1). The proline concentrations significantly increased by about 7.04- and 5.32-fold under salt (216.40 ± 91.23 µg g−1) and S-D-H (163.54 ± 10.41 µg g−1) stresses, respectively, compared to the control plants, with no significant changes in the other treatments.
3. Differential Physio-Biochemical and Metabolic Responses of Peanut (Arachis hypogaea L.) under Multiple Abiotic Stress Conditions
Climate change and global warming have increased the severity and frequency of extreme climatic conditions. Plant biotechnology researchers often work on individual abiotic stresses, but plants usually experience multiple abiotic stresses at once or sequentially in the field, inducing different responses to individual stresses. Plants selectively change physio-biochemical properties and metabolic pathways to adjust to the adverse effects of these abiotic stresses. The combination of some abiotic stresses such as drought and heat may produce conflicting responses in plants. Plant adaptation to abiotic stress is shaped by the environment that is confronting the plant. Consequently, changes in environmental conditions can alter the molecular, biochemical, and physiological responses in plants.
China is the largest producer of peanut (17.99 million metric tons) followed by India (6.70 million metric tons) and the USA (2.79 million metric tons); however, the USA produces the highest yields (4.27 metric tons per ha), followed by China (3.79 metric tons per ha) and India (1.12 metric tons per ha) [30]. Peanut is an important cash crop worldwide, producing India’s third-most consumed edible oil. Various abiotic stresses are the key reason for reduced yields in India [31].
Cluster computing and yield simulation suggest that climate change will reduce peanut production by 2.3–33.7% in India [32]. In this study, different abiotic stress combinations were used to simulate possible future climatic conditions and their effects on peanut plants. This study investigated the effect of individual and combined abiotic stresses on the peanut’s physio-biochemical and metabolomic responses, elucidating their effect on the complex metabolic networks and pathways and metabolite accumulation. In addition, multivariate correlation analysis of the physio-biochemical and metabolomic parameters was used to understand the molecular mechanism of abiotic stress responses in peanut plants.
ROS are an integral part of plant sensing and signaling [33][34]. Plants in unstressed environments maintain a delicate balance between ROS production and scavenging, which are involved in cellular signaling. Exposure to abiotic stress causes excess ROS production in plant cells, damaging cellular membranes [35]. DAB (brown precipitate) and NBT (blue precipitate) histochemical staining produce visible staining in leaf explants by reacting with accumulated peroxide and free oxygen radicals, respectively [36]. DAB staining of peanut leaves suggested that combined stresses such as S-H, S-D, D-H, and S-D-H significantly induced higher peroxide radical formation than the individual stresses. Similarly, NBT staining showed that free oxygen radicals accumulated significantly under S-D, S-C, D-H, S-D-H, and S-D-C stress compared to the control and individual stresses. Thus, the combined stresses accumulated more ROS in peanut plants than the individual stresses. Abiotic stresses cause lipid peroxidation of membranes leading to cellular membrane damage and leakage [31]. In Sesamum indicum cv. Orhangazi, salt stress significantly increased the lipid peroxidation [37]. In contrast, lipid peroxidation did not significantly change in the individual stress treatments. Interestingly, lipid peroxidation increased in peanut under S-H and S-D-H stress, suggesting the presence of strong antioxidant machinery.
The antioxidative enzyme system of plants scavenges excess ROS and restores homeostasis. A study on peanut plants reported that CAT activity in the leaf tissue decreased by up to 52% under drought stress compared to the control [22]. Similarly, in the study, the CAT activity decreased by 72% under drought stress, more so when combined with salt (S-D, 84%), heat (D-H, 85%), or salt and heat (S-D-H, 94%) stress. Similar results were reported for Hedychium plants, where combined drought and heat stress decreased the CAT activity in leaf samples [11]. In contrast, combined cold and drought (D-C, 56%) and salt, drought, and cold (S-D-C, 48%) stress significantly increased CAT activity. Under salt stress, SOD and APX activities decreased by 50–70% in peanut plants [38]. In contrast, the SOD activity increased, and APX activity remained stable under salt stress in the present study compared to the control. The different SOD and APX responses may be related to differences in the genotype and stress duration; for example, 48 h stress with GG20 cultivar in this study compared to 96 h stress with Luhua14 cultivar [38]. The APX activity almost doubled under S-C and S-D-C stress. Glutathione reductase (GR) activity increased significantly in green spurge (Euphorbia esula) under individual drought and cold stresses compared to the control [39]. In the study, the GR activity increased under all individual and combined stresses, but more so for the combined stresses that included salt (S-C, S-H, S-D-H).
Plant biochemical constituents such as amino acids, polyphenol, sugars, and starch play important roles in osmotic homeostasis, sensing and signaling, and growth during abiotic stress [40]. The total soluble sugar concentration increased in soybean leaves after long-term drought stress [41]. Similarly, in peanut plants, the sugar and starch concentrations increased under salt, drought, and S-D stress, perhaps acting as osmoprotectants and helping maintain turgor pressure and membrane stability. In Arabidopsis, the accumulation of sugars, amino acids, and polyphenols under drought stress drives ion and osmotic homeostasis [42]. In peanut leaves, FAA concentrations increased in all individual and combined stress treatments except for those that were related to cold stress, indicating that cold stress ameliorates the effect of other concurrent abiotic stresses.
Proline is a particularly important osmoprotectant for plant abiotic stress tolerance [43]. Transgenic tobacco plants that were overexpressing the proline biosynthesis gene pyrroline 5-carboxylate synthetase, had better drought, heat, and drought-heat sequential stress tolerance than control plants, indicating the role of proline accumulation in abiotic stress tolerance [44]. The overexpression of proline-synthesizing genes or the exogenous application of proline enhanced the salt tolerance in plants [45]. In soybean plants, the overexpression of transcription factor GmDREB6 led to proline accumulation and enhanced salinity tolerance [46]. Likewise, proline concentrations, measured by calorimetry, significantly increased under salt, S-H, D-H, S-D, and S-D-H stress, indicating its involvement in plant defense mechanisms against abiotic stress. Especially, significantly more proline accumulated under S-D than individual salt or drought stress, revealing the destructive nature of the combined stress.
In this study, the PCA biplot of metabolites showed that salt stress significantly increased the proline content in peanut plants, confirming its importance for salt stress tolerance. Relatedly, the proline concentration increased in switchgrass by overexpressing proline-synthesizing enzyme genes under multiple individual abiotic stresses such as salt, heat, drought, and cold [47]. Similarly, the PCA biplot shows that proline significantly correlated with D-H stress in this study, suggesting that plants accumulate high proline content under heat and osmotic stress. Amino acid homeostasis plays an essential role in the tolerance mechanism through protein synthesis or degradation [48]. Individual stresses accumulated different amino acids [cold (leucine and valine), drought (asparagine and isoleucine), and heat (glutamic acid and serine)]. Combined stresses that included salt stress (S-D and S-D-H) significantly correlated with isoleucine and tyrosine, whereas D-H significantly correlated with proline, valine, threonine, tryptophan, and phenylalanine.
Polyphenols are a group of plant secondary metabolites with various functions, such as growth and regulation, abiotic stress tolerance, UV-B radiation endurance, and color, sensory, and antioxidant properties [49]. The total polyphenol content and antioxidant activity in green barley increased under combined drought and light stress [50]. Similarly, in peanut plants, drought-related stresses (drought, S-D, D-H, S-D-H) increased the polyphenol content, more so for the combined stresses.
Potato cultivars, Burbank and Unica, had low total chlorophyll concentrations after salt, drought, and combined salt and drought stress [51]. In contrast, salt stress did not affect the chlorophyll content of Suaeda fruticosa leaves [52]. Similarly, in peanut plants, only slight (insignificant) changes in pigment content occurred under the individual stresses; however, significant increases occurred under the combined stresses (S-D, D-C, S-D-H, S-D-C). The strong antioxidant machinery and improved osmotic homeostasis in peanut plants might have helped increase the pigment concentration under combined stress. The leaf RWC decreased significantly under drought and D-H stress in two Himalayan plant species, tagar-ganthoda (Valeriana jatamansi) and spiked ginger lily (Hedychium spicatum) [11]. Likewise, in peanut plants, RWC decreased significantly under drought stress, with additive effects that were observed under combined stresses that included drought (S-D, D-H, and S-D-H). However, cold stress ameliorated the effect of drought, with only a slight decrease in RWC under D-C and S-D-C stress. Electrolyte leakage of tall fescue (Festuca arundinacea) increased significantly under individual salt, drought, and heat stresses [53]. Surprisingly, in peanut, EL only increased in the treatments containing heat stress (heat, S-H, D-H, and S-D-H), which may be due to the high membrane stability that was observed in all the heat-treated plants, preventing EL from cells.
Plants change their metabolome to acclimatize to their surrounding environment. Small changes in environmental parameters can trigger changes in metabolic pathways, leading to the synthesis, accumulation, or degradation of different metabolites in cells. Combined stresses alter the metabolome of plants more than individual stresses. Analysis of the effect of combined stress on the peanut metabolome using PCA biplots, pathway enrichment, heatmaps, PLS-DA, and correlation analysis offered valuable insights into abiotic stress tolerance mechanisms.
The stress treatments significantly altered the TCA and urea cycles and their associated amino acid biosynthesis pathway intermediates. The exogenous application of citric acid (TCA intermediate) increased abiotic stress tolerance by improving ROS homeostasis, photosynthetic rates, and osmoregulation [54]. In the study, citric acid concentration decreased in all the stress treatments (individual and combined) except heat, whereas cis-aconitate concentration increased in most heat-related stresses. These results indicate that citric acid was not formed from pyruvate or rapidly converted to cis-aconitate, the next intermediate in the TCA cycle. Increasing the pyruvate concentration increased valine, leucine, and isoleucine biosynthesis as the concentration of these amino acids increased under abiotic stress. Increased cis-aconitate leads to increased GABA or 4-aminobutanoate concentrations, increasing the production of glutamic acid, glycine, and serine.
Recently, the role of urea cycle intermediates such as ornithine, aspartate, arginine, and citrulline was reported in plant abiotic stress tolerance mechanisms [55][56][57][58]. Engineered Arabidopsis overproducing ornithine showed enhanced tolerance to salt and drought stress, which may be due to the increased ornithine producing arginine and aspartate, intermediates of proline biosynthesis [56]. In contrast, the urea concentration decreased significantly in peanut plants under all abiotic stress treatments except for drought and S-D, indicating reduced ornithine co-production. An Arabidopsis mutant of arginine synthase had lower arginine concentrations leading to enhanced byproducts, such as polyamines, NO, and citrulline, involved in abiotic stress tolerance [57]. In peanut plants, the arginine precursor aspartate decreased under most abiotic stresses, increasing the production of asparagine, phenylalanine, tyrosine, alanine, lysine, and proline.
Abiotic stresses significantly affected the plant metabolome by altering metabolic pathways. Pathway enrichment analysis identified the most affected metabolic pathways in peanut plants under individual and combined abiotic stresses. Salt, drought, and salt-drought stress differentially accumulated free amino acids in mangrove (Avicennia marina) [59]. Likewise, individual and combined abiotic stresses significantly affected amino acids, especially those in the valine, leucine, and isoleucine biosynthesis pathways. Transcriptome analysis of Chinese cabbage (Brassica rapa) under drought stress showed differential expression of transcription factors and acclimation response for glucosinolate metabolism [60]. Similarly, individual and combined stresses significantly affected glucosinolate metabolism in peanut plants. Galactose metabolism led to the production of ascorbic acid, a strong antioxidant during abiotic stress. The manipulation of the galactose metabolism pathway and the overproduction of ascorbic acid enhanced the abiotic stress tolerance in rice [61]. Peanut plants under abiotic stress also had an altered galactose metabolism pathway. Similarly, the sugar metabolism pathway changes to provide abiotic stress tolerance [62]. In peanut plants, the pathways related to sugar metabolism, including pentose and glucuronate interconversion, C5 branched dibasic acid metabolism, and pantothenate and Co-A biosynthesis pathways significantly changed to cope with abiotic stress. Drought stress enhanced indole alkaloid biosynthesis in the medicinally important plant periwinkle (Catharanthus roseus var. rosea) [63]. Similarly, indole alkaloid biosynthesis increased in peanut plants, enhancing antioxidant activities during abiotic stress.
This entry is adapted from the peer-reviewed paper 10.3390/ijms23020660
References
- Patel, J.; Mishra, A. Genome editing: Advances and prospects. In Plant Biotechnology: Progress in Genomic Era; Khurana, S.M.P., Gaur, R.K., Eds.; Springer: Singapore, 2019; pp. 147–174.
- Choudhury, F.K.; Rivero, R.M.; Blumwald, E.; Mittler, R. Reactive oxygen species, abiotic stress and stress combination. Plant J. 2017, 90, 856–867.
- Gill, R.A.; Ahmar, S.; Ali, B.; Saleem, M.H.; Khan, M.U.; Zhou, W.; Liu, S. The Role of Membrane Transporters in Plant Growth and Development, and Abiotic Stress Tolerance. Int. J. Mol. Sci. 2021, 22, 12792.
- Imran, M.; Sun, X.; Hussain, S.; Ali, U.; Rana, M.S.; Rasul, F.; Saleem, M.H.; Moussa, M.G.; Bhantana, P.; Afzal, J.; et al. Molybdenum-Induced Effects on Nitrogen Metabolism Enzymes and Elemental Profile of Winter Wheat (Triticum aestivum L.) Under Different Nitrogen Sources. Int. J. Mol. Sci. 2019, 20, 3009.
- Kamran, M.; Parveen, A.; Ahmar, S.; Malik, Z.; Hussain, S.; Chattha, M.S.; Saleem, M.H.; Adil, M.; Heidari, P.; Chen, J.-T. An Overview of Hazardous Impacts of Soil Salinity in Crops, Tolerance Mechanisms, and Amelioration through Selenium Supplementation. Int. J. Mol. Sci. 2020, 21, 148.
- Nawaz, M.; Wang, X.; Saleem, M.H.; Khan, M.H.U.; Afzal, J.; Fiaz, S.; Ali, S.; Ishaq, H.; Khan, A.H.; Rehman, N.; et al. Deciphering Plantago ovata Forsk Leaf Extract Mediated Distinct Germination, Growth and Physio-Biochemical Improvements under Water Stress in Maize (Zea mays L.) at Early Growth Stage. Agronomy 2021, 11, 1404.
- Alam, H.; Khattak, J.Z.K.; Ksiksi, T.S.; Saleem, M.H.; Fahad, S.; Sohail, H.; Ali, Q.; Zamin, M.; El-Esawi, M.A.; Saud, S. Negative impact of long-term exposure of salinity and drought stress on native Tetraena mandavillei L. Physiol. Plant. 2020, 172, 1336–1351.
- Ghafar, M.A.; Akram, N.A.; Saleem, M.H.; Wang, J.; Wijaya, L.; Alyemeni, M.N. Ecotypic Morphological and Physio-Biochemical Responses of Two Differentially Adapted Forage Grasses, Cenchrus ciliaris L. and Cyperus arenarius Retz. to Drought Stress. Sustainability 2021, 13, 8069.
- Cohen, I.; Zandalinas, S.I.; Fritschi, F.B.; Sengupta, S.; Fichman, Y.; Azad, R.K.; Mittler, R. The impact of water deficit and heat stress combination on the molecular response, physiology, and seed production of soybean. Physiol. Plant. 2021, 172, 41–52.
- Patel, J.; Mishra, A. Plant aquaporins alleviate drought tolerance in plants by modulating cellular biochemistry, root-architecture, and photosynthesis. Physiol. Plant. 2021, 172, 1030–1044.
- Pandey, V.; Tiwari, D.C.; Dhyani, V.; Bhatt, I.D.; Rawal, R.S.; Nandi, S.K. Physiological and metabolic changes in two Himalayan medicinal herbs under drought, heat and combined stresses. Physiol. Mol. Biol. Plants 2021, 27, 1523–1538.
- Yasmin, H.; Bano, A.; Wilson, N.L.; Nosheen, A.; Naz, R.; Hassan, M.N.; Ilyas, N.; Saleem, M.H.; Noureldeen, A.; Ahmad, P.; et al. Drought-tolerant Pseudomonas sp. showed differential expression of stress-responsive genes and induced drought tolerance in Arabidopsis thaliana. Physiol. Plant. 2021.
- Sinha, R.; Fritschi, F.B.; Zandalinas, S.I.; Mittler, R. The impact of stress combination on reproductive processes in crops. Plant Sci. 2021, 311, 111007.
- Fábián, A.; Sáfrán, E.; Szabó-Eitel, G.; Barnabás, B.; Jäger, K. Stigma Functionality and Fertility Are Reduced by Heat and Drought Co-stress in Wheat. Front. Plant Sci. 2019, 10, 244.
- Liu, X.; Wang, X.; Wang, X.; Gao, J.; Luo, N.; Meng, Q.; Wang, P. Dissecting the critical stage in the response of maize kernel set to individual and combined drought and heat stress around flowering. Environ. Exp. Bot. 2020, 179, 104213.
- Patel, M.K.; Mishra, A.; Jha, B. Untargeted metabolomics of halophytes. In Marine Omics: Principles and Applications; Kim, S.K., Ed.; Taylor & Francis Group, CRC Press: Boca Raton, FL, USA, 2016; pp. 309–325.
- Lawas, L.M.F.; Li, X.; Erban, A.; Kopka, J.; Jagadish, S.V.K.; Zuther, E.; Hincha, D.K. Metabolic responses of rice cultivars with different tolerance to combined drought and heat stress under field conditions. GigaScience 2019, 8, giz050.
- Correia, B.; Hancock, R.D.; Amaral, J.; Gomez-Cadenas, A.; Valledor, L.; Pinto, G. Combined Drought and Heat Activates Protective Responses in Eucalyptus globulus That Are Not Activated When Subjected to Drought or Heat Stress Alone. Front. Plant Sci. 2018, 9, 819.
- Sprenger, H.; Erban, A.; Seddig, S.; Rudack, K.; Thalhammer, A.; Le, M.Q.; Walther, D.; Zuther, E.; Köhl, K.I.; Kopka, J.; et al. Metabolite and transcript markers for the prediction of potato drought tolerance. Plant Biotechnol. J. 2018, 16, 939–950.
- Templer, S.E.; Ammon, A.; Pscheidt, D.; Ciobotea, O.; Schuy, C.; McCollum, C.; Sonnewald, U.; Hanemann, A.; Förster, J.; Ordon, F.; et al. Metabolite profiling of barley flag leaves under drought and combined heat and drought stress reveals metabolic QTLs for metabolites associated with antioxidant defense. J. Exp. Bot. 2017, 68, 1697–1713.
- Jha, B.; Mishra, A.; Chaturvedi, A. Engineering stress tolerance in peanut (Arachis hypogaea L.). In Genetically Modified Organisms (GMO) Foods: Production, Regulation and Public Health; Watson, R., Preedy, V.R., Eds.; Academic Press, Elsevier: San Diego, CA, USA, 2016; pp. 305–311.
- Azevedo Neto, A.D.; Nogueira, R.J.M.C.; Melo Filho, P.A.; Santos, R.C. Physiological and biochemical responses of peanut genotypes to water deficit. J. Plant Interact. 2010, 5, 1–10.
- Gundaraniya, S.A.; Ambalam, P.S.; Tomar, R.S. Metabolomic Profiling of Drought-Tolerant and Susceptible Peanut (Arachis hypogaea L.) Genotypes in Response to Drought Stress. ACS Omega 2020, 5, 31209–31219.
- Sharma, S.; Choudhary, B.; Yadav, S.; Mishra, A.; Mishra, V.K.; Chand, R.; Chen, C.; Pandey, S.P. Metabolite profiling identified pipecolic acid as an important component of peanut seed resistance against Aspergillus flavus infection. J. Hazard. Mater. 2021, 404, 124155.
- Singh, N.; Mishra, A.; Jha, B. Over-expression of the peroxisomal ascorbate peroxidase (SbpAPX) gene cloned from halophyte Salicornia brachiata confers salt and drought stress tolerance in transgenic tobacco. Mar. Biotechnol. 2014, 16, 321–332.
- Singh, N.; Mishra, A.; Jha, B. Ectopic over-expression of peroxisomal ascorbate peroxidase (SbpAPX) gene confers salt stress tolerance in transgenic peanut (Arachis hypogaea). Gene 2014, 547, 119–125.
- Cui, F.; Sui, N.; Duan, G.; Liu, Y.; Han, Y.; Liu, S.; Wan, S.; Li, G. Identification of Metabolites and Transcripts Involved in Salt Stress and Recovery in Peanut. Front. Plant Sci. 2018, 9, 217.
- Raval, S.S.; Mahatma, M.K.; Chakraborty, K.; Bishi, S.K.; Singh, A.L.; Rathod, K.J.; Jadav, J.K.; Sanghani, J.M.; Mandavia, M.K.; Gajera, H.P.; et al. Metabolomics of groundnut (Arachis hypogaea L.) genotypes under varying temperature regimes. Plant Growth Regul. 2017, 84, 493–505.
- Jha, R.K.; Mishra, A. Introgression of SbERD4 gene encodes an early-responsive dehydration-stress protein that confers tolerance against different types of abiotic stresses in transgenic tobacco. Cells 2022, 11, 62.
- World Agricultural Production. Available online: https://www.fas.usda.gov/data/world-agricultural-production (accessed on 13 December 2021).
- Tiwari, V.; Chaturvedi, A.K.; Mishra, A.; Jha, B. Introgression of the SbASR-1 Gene Cloned from a Halophyte Salicornia brachiata Enhances Salinity and Drought Endurance in Transgenic Groundnut (Arachis hypogaea) and Acts as a Transcription Factor. PLoS ONE 2015, 10, e0131567.
- Kadiyala, M.D.M.; Nedumaran, S.; Padmanabhan, J.; Gumma, M.K.; Gummadi, S.; Srigiri, S.R.; Robertson, R.; Whitbread, A. Modeling the potential impacts of climate change and adaptation strategies on groundnut production in India. Sci. Total Environ. 2021, 776, 145996.
- Ashraf, M.A.; Akbar, A.; Askari, S.H.; Iqbal, M.; Rasheed, R.; Hussain, I. Recent Advances in Abiotic Stress Tolerance of Plants through Chemical Priming: An Overview. In Advances in Seed Priming; Rakshit, A., Singh, H., Eds.; Springer: Singapore, 2018.
- Ashraf, M.A.; Asma, H.F.; Iqbal, M. Exogenous menadione sodium bisulfite mitigates specific ion toxicity and oxidative damage in salinity-stressed okra (Abelmoschus esculentus Moench). Acta Physiol. Plant. 2019, 41, 187.
- Patel, J.; Mishra, A. Role of sodium proton antiporters in cellular homeostasis of plants under abiotic stress conditions. In Transporters and Plant Osmotic Stress; Roychoudhury, A., Tripathi, D.K., Deshmukh, R., Eds.; Academic Press: San Diego, CA, USA; Elsevier: San Diego, CA, USA, 2021; pp. 273–290.
- Jha, R.K.; Patel, J.; Patel, M.K.; Mishra, A.; Jha, B. Introgression of a novel cold and drought regulatory-protein encoding CORA-like gene, SbCDR, induced osmotic tolerance in transgenic tobacco. Physiol. Plant. 2021, 172, 1170–1188.
- Koca, H.; Bor, M.; Özdemir, F.; Türkan, İ. The effect of salt stress on lipid peroxidation, antioxidative enzymes and proline content of sesame cultivars. Environ. Exp. Bot. 2007, 60, 344–351.
- Sui, N.; Wang, Y.; Liu, S.; Yang, Z.; Wang, F.; Wan, S. Transcriptomic and Physiological Evidence for the Relationship between Unsaturated Fatty Acid and Salt Stress in Peanut. Front. Plant Sci. 2018, 9, 7.
- Anderson, J.V.; Davis, D.G. Abiotic stress alters transcript profiles and activity of glutathione S-transferase, glutathione peroxidase, and glutathione reductase in Euphorbia esula. Physiol. Plant. 2004, 120, 421–433.
- Jha, R.K.; Patel, J.; Mishra, A.; Jha, B. Introgression of halophytic salt stress-responsive genes for developing stress tolerance in crop plants. In Halophytes and Climate Change: Adaptive Mechanisms and Potential Uses; Hasanuzzaman, M., Shabala, S., Fujita, M., Eds.; CABI: Wallingford, UK, 2019; pp. 275–286.
- Mutava, R.N.; Prince, S.J.K.; Syed, N.H.; Song, L.; Valliyodan, B.; Chen, W.; Nguyen, H.T. Understanding abiotic stress tolerance mechanisms in soybean: A comparative evaluation of soybean response to drought and flooding stress. Plant Physiol. Biochem. 2015, 86, 109–120.
- Sperdouli, I.; Moustakas, M. Interaction of proline, sugars, and anthocyanins during photosynthetic acclimation of Arabidopsis thaliana to drought stress. J. Plant Physiol. 2012, 169, 577–585.
- Mfarrej, M.F.B.; Wang, X.; Saleem, M.H.; Hussain, I.; Rasheed, R.; Ashraf, M.A.; Iqbal, M.; Chattha, M.S.; Alyemeni, M.N. Hydrogen sulphide and nitric oxide mitigate the negative impacts of waterlogging stress on wheat (Triticum aestivum L.). Plant Biol. 2021.
- Dobra, J.; Motyka, V.; Dobrev, P.; Malbeck, J.; Prasil, I.T.; Haisel, D.; Gaudinova, A.; Havlova, M.; Gubis, J.; Vankova, R. Comparison of hormonal responses to heat, drought and combined stress in tobacco plants with elevated proline content. J. Plant Physiol. 2010, 167, 1360–1370.
- El Moukhtari, A.; Cabassa-Hourton, C.; Farissi, M.; Savouré, A. How Does Proline Treatment Promote Salt Stress Tolerance During Crop Plant Development? Front. Plant Sci. 2020, 11, 1127.
- Nguyen, Q.H.; Vu, L.T.K.; Nguyen, L.T.N.; Pham, N.T.T.; Nguyen, Y.T.H.; Le, S.V.; Chu, M.H. Overexpression of the GmDREB6 gene enhances proline accumulation and salt tolerance in genetically modified soybean plants. Sci. Rep. 2019, 9, 19663.
- Guan, C.; Huang, Y.H.; Cui, X.; Liu, S.J.; Zhou, Y.Z.; Zhang, Y.W. Overexpression of gene encoding the key enzyme involved in proline-biosynthesis (PuP5CS) to improve salt tolerance in switchgrass (Panicum virgatum L.). Plant Cell Rep. 2018, 37, 1187–1199.
- Hildebrandt, T.M. Synthesis versus degradation: Directions of amino acid metabolism during Arabidopsis abiotic stress response. Plant Mol. Biol. 2018, 98, 121–135.
- Naikoo, M.I.; Dar, M.I.; Raghib, F.; Jaleel, H.; Ahmad, B.; Raina, A.; Khan, F.A.; Naushin, F. Role and Regulation of Plants Phenolics in Abiotic Stress Tolerance. In Plant Signaling Molecules; Khan, M.I., Reddy, P.S., Ferrante, A., Khan, N.A., Eds.; Woodhead Publishing: Cambridge, UK, 2019; pp. 157–168.
- Kowalczewski, P.L.; Radzikowska, D.; Ivanisova, E.; Szwengiel, A.; Kacaniova, M.; Sawinska, Z. Influence of Abiotic Stress Factors on the Antioxidant Properties and Polyphenols Profile Composition of Green Barley (Hordeum vulgare L.). Int. J. Mol. Sci. 2020, 21, 397.
- Demirel, U.; Morris, W.L.; Ducreux, L.J.M.; Yavuz, C.; Asim, A.; Tindas, I.; Campbell, R.; Morris, J.A.; Verrall, S.R.; Hedley, P.E.; et al. Physiological, Biochemical, and Transcriptional Responses to Single and Combined Abiotic Stress in Stress-Tolerant and Stress-Sensitive Potato Genotypes. Front. Plant Sci. 2020, 11, 169.
- Bankaji, I.; Sleimi, N.; López-Climent, M.F.; Perez-Clemente, R.M.; Gomez-Cadenas, A. Effects of Combined Abiotic Stresses on Growth, Trace Element Accumulation, and Phytohormone Regulation in Two Halophytic Species. J. Plant Growth Regul. 2014, 33, 632–643.
- Xie, Y.; Sun, X.; Feng, Q.; Luo, H.; Wassie, M.; Amee, M.; Amombo, E.; Chen, L. Comparative physiological and metabolomic analyses reveal mechanisms of Aspergillus aculeatus-mediated abiotic stress tolerance in tall fescue. Plant Physiol. Biochem. 2019, 142, 342–350.
- Tahjib-Ul-Arif, M.; Zahan, M.I.; Karim, M.M.; Imran, S.; Hunter, C.T.; Islam, M.S.; Mia, M.A.; Hannan, M.A.; Rhaman, M.S.; Hossain, M.A.; et al. Citric Acid-Mediated Abiotic Stress Tolerance in Plants. Int. J. Mol. Sci. 2021, 22, 7235.
- Blume, C.; Ost, J.; Mühlenbruch, M.; Peterhänsel, C.; Laxa, M. Low CO2 induces urea cycle intermediate accumulation in Arabidopsis thaliana. PLoS ONE 2019, 14, e0210342.
- Kalamaki, M.S.; Merkouropoulos, G.; Kanellis, A.K. Can ornithine accumulation modulate abiotic stress tolerance in Arabidopsis? Plant Signal. Behav. 2009, 4, 1099–1101.
- Shi, H.; Ye, T.; Chen, F.; Cheng, Z.; Wang, Y.; Yang, P.; Zhang, Y.; Chan, Z. Manipulation of arginase expression modulates abiotic stress tolerance in Arabidopsis: Effect on arginine metabolism and ROS accumulation. J. Exp. Bot. 2013, 64, 1367–1379.
- Song, Q.; Joshi, M.; DiPiazza, J.; Joshi, V. Functional Relevance of Citrulline in the Vegetative Tissues of Watermelon during Abiotic Stresses. Front. Plant Sci. 2020, 11, 512.
- Ravi, S.; Young, T.; Macinnis-Ng, C.; Nyugen, T.V.; Duxbury, M.; Alfaro, A.C.; Leuzinger, S. Untargeted metabolomics in halophytes: The role of different metabolites in New Zealand mangroves under multi-factorial abiotic stress conditions. Environ. Exp. Bot. 2020, 173, 103993.
- Eom, S.H.; Baek, S.-A.; Kim, J.K.; Hyun, T.K. Transcriptome Analysis in Chinese Cabbage (Brassica rapa ssp. pekinensis) Provides the Role of Glucosinolate Metabolism in Response to Drought Stress. Molecules 2018, 23, 1186.
- Zhang, G.Y.; Liu, R.R.; Zhang, C.Q.; Tang, K.X.; Sun, M.F.; Yan, G.H.; Liu, Q.Q. Manipulation of the Rice L-Galactose Pathway: Evaluation of the Effects of Transgene Overexpression on Ascorbate Accumulation and Abiotic Stress Tolerance. PLoS ONE 2015, 10, e0125870.
- Rosa, M.; Prado, C.; Podazza, G.; Interdonato, R.; González, J.A.; Hilal, M.; Prado, F.E. Soluble sugars. Plant Signal. Behav. 2009, 4, 388–393.
- Jaleel, C.A.; Gopi, R.; Manivannan, P.; Gomathinayagam, M.; Sridharan, R.; Panneerselvam, R. Antioxidant potential and indole alkaloid profile variations with water deficits along different parts of two varieties of Catharanthus roseus. Colloids Surf. B Biointerfaces 2008, 62, 312–318.
This entry is offline, you can click here to edit this entry!
