Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Pharmacology & Pharmacy
3-iodothyronamine (T1AM) and 3-iodothyroacetic acid (TA1) are thyroid-hormone-related compounds endowed with pharmacological activity through mechanisms that remain elusive. Some evidence suggests that they may have redox features.
- 3-iodothyronamine
- 3-iodothyroacetic acid
- antioxidant
- sirtuin-1
1. Introduction
Both 3-iodothyronamine (T1AM) and its oxidative metabolite, 3-iodothyroacetic acid (TA1) are endogenous compounds circulating in mammals [1]. Due to the fact that their structure is somehow related to thyroid hormones (THs; i.e., T3 and T4), it was hypothesized they represented end metabolites of THs and possible players of the non-genomic effects of T3. However, their biosynthetic pathway and physio-pathological role remain elusive. Despite this, T1AM and TA1 show pharmacological features in rodents, which suggests the merit to be exploited for their potential clinical effectiveness.
T1AM is considered a multi-target compound [2] and, even more than TA1, its mechanism of action remains mostly elusive and undefined [3]. Overall, the effects of T1AM and TA1, such as their rapid onset, high potency, non-linear dose–effect relationship and signaling, indicate that their mechanisms may include additional or alternative pathways to the recognition of a target. In this respect, some clues suggested that T1AM and TA1 modify the redox status of the system studied.
In fact, at some settings, T1AM’s effects are mediated, at least in part, by the formation of TA1, a product of T1AM oxidative deamination by the mitochondrial monoamine oxidase type B (MAO-B) and/or membrane-bound semicarbazide-sensitive amine oxidases (SSAO). These enzymes produce reactive oxygen species (ROS), which have been found to be casually involved in the pathogenesis of different diseases [4][5][6]. Further, T1AM, but also TA1, protect against excitotoxicity and ischemia-reperfusion injury and ROS-generating conditions, in both in vivo and ex vivo settings [7][8][9][10]. Moreover, Ghanian et al. [11] first reported that T1AM dose-dependently increased the redox ratio of the renal but not cardiac mitochondria of mice. Furthermore, Sacripanti et al. [12] and Assadi-Porter et al. [13] reported that T1AM modulated sirtuin (SIRTs) levels, including sirtuin-1 (SIRT1), a mitochondrial NAD+-dependent deacethylase playing a crucial role in controlling genome stability and considered a target and/or sensor of cell ROS levels [14]. Consistently with this latter, the activation of SIRT1 is a fingerprint signaling of known antioxidants [15][16]. Lastly, in their recent work, [17] researchers reported that brown adipocytes (BAs) differentiated in the presence of 20 nM T1AM resulted in a cell population (M + T1AM cells) showing a pro-oxidant metabolism compared to cells not exposed to T1AM (M cells).
Drugs acting on the redox status may act as pro- or anti-oxidants or ROS scavengers. These molecules achieve their effects by means of their chemical structure, acting as reducing agents, detoxifying ROS, or “indirectly” preventing ROS formation. This latter class includes compounds controlling the expression/activity of ROS-generating or ROS-detoxifying proteins [18][19]. In this respect, less is known about the antioxidant/pro-oxidant capacity of T1AM and TA1 and whether they can exert control on the expression/activity of the MAO and SSAO, enzymes included in the pharmacokinetics of T1AM and on the antioxidant deacetylase SIRT1.
2. T1AM and TA1 Showed Ferric-Reducing Capacity and Superoxide and Hydroxyl Radical Scavenging Activities
The ferric reducing activity power (FRAP) of T1AM and TA1. As positive controls, resveratrol (RESV) and α-tocopherol (α-TOC) were used.
As shown in Figure 1, panel a, the FRAP activity of TA1 and T1AM was evident at concentrations as low as 0.1 μM and it was higher, at 2000 nM (Figure 2, panel b), than that measured for RESV and α-TOC (°°° p < 0.001 vs. RESV, *** p < 0.001 vs. α-TOC).
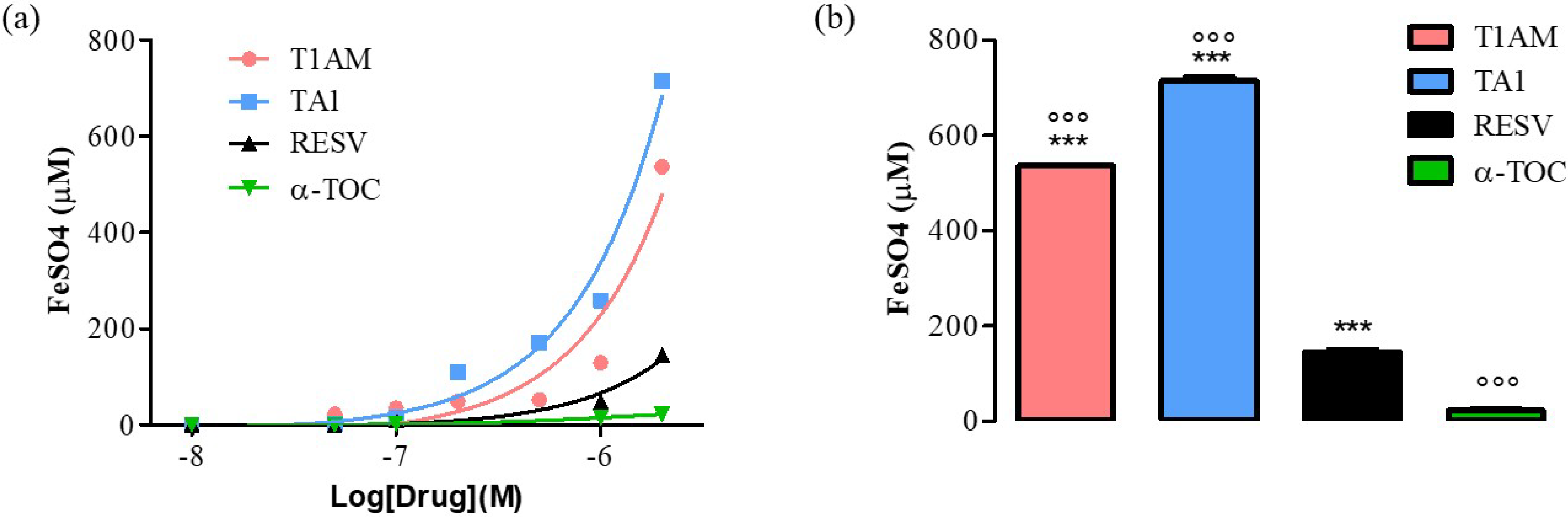
Figure 1. (a) Curves produced during the FRAP assay with T1AM, TA1 (10, 50, 100, 200, 500, 1000, 2000 nM), RESV, α-TOC (10, 50, 100, 1000, 2000 nM). (b) Bar graph showing FeSO4 equivalent (µM) for T1AM, TA1, RESV, and α-TOC at 2000 nM. All experiments were performed in triplicate. Data are expressed as mean ± SEM; °°° p < 0.001 vs. RESV, *** p < 0.001 vs. α-TOC.
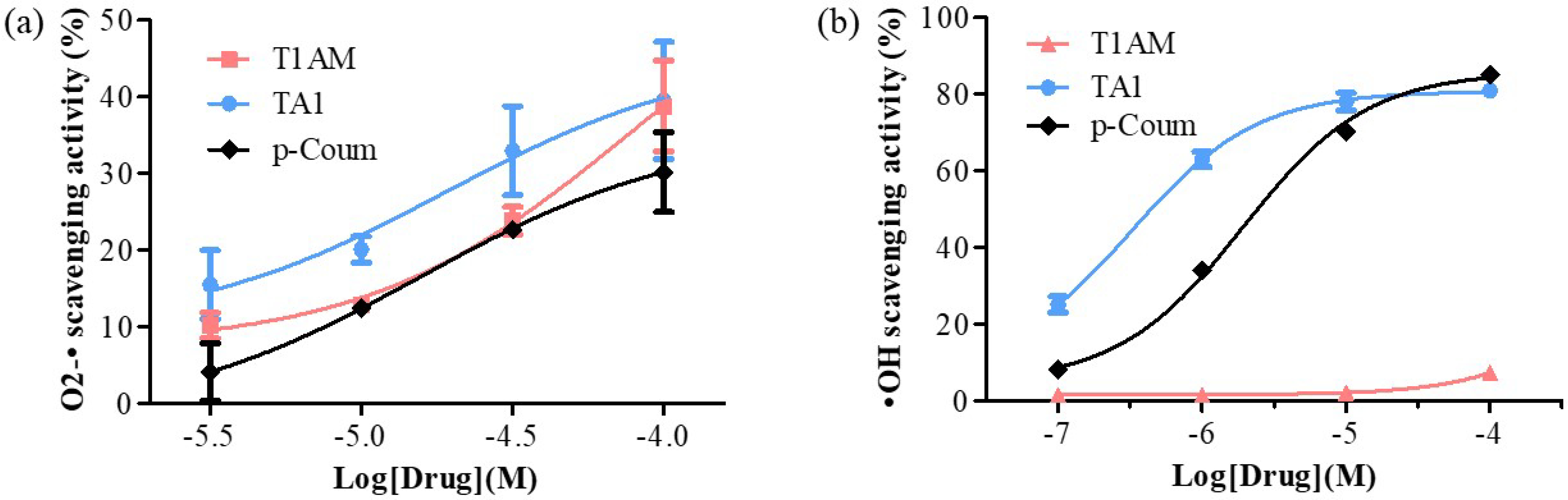
Figure 2. Determination of (a) O2-• and (b) •OH scavenging activities of T1AM, TA1, and p-Coum (3, 10, 30, and 100 μM and 0.1, 1, 10, and 100 μM, respectively). All experiments were performed in triplicate. Data are expressed as mean ± SEM.
T1AM and TA1 also showed free radical scavenging features with some interesting peculiarities. In fact, TA1 showed both superoxide and hydroxyl radical scavenging activities (pEC50 ± SEM of −4.72 ± 0.78 and −6.49 ± 0.10 respectively; Figure 2a,b) with the potency towards the hydroxyl radicals being significantly higher (*** p < 0.001) than that measured for the p-coumaric acid (p-Coum) (pEC50 ± SEM −5.71 ± 0.05; Figure 2b).
Instead, their results clearly showed that T1AM had only the capacity to scavenge superoxide radicals (pEC50 ± SEM of −4.12 ± 0.54; Figure 2a) with a potency similar to that of p-Coum (pEC50 −4.80 ± 0.63; Figure 2a).
The FRAP activity of T1AM and of TA1 and their ROS scavenging features indicated that both compounds, at high pro-oxidant conditions, behave as antioxidants.
3. T1AM and TA1 Modify the Expression and Activity Levels of Pro- and Antioxidant Cell Proteins
Researchers then investigated the expression and activity levels of pro- and antioxidant proteins, including UCP-1, MAO (A and B), SSAO, and SIRT1 in M, M + T1AM, and M + TA1 cells.
3.1. Evaluation of UCP-1 Protein and Amine Oxidases mRNA Levels in M + T1AM and M + TA1 Cells
Researchers previously demonstrated that UCP-1 protein levels were similar in M + T1AM and M cells [17]. Instead, in this study, as reported in Figure 3 (panels a and b), the M + TA1 cells showed significantly lower UCP-1 protein expression (Figure 3, panel a, b; ** p < 0.01) than the M cells.
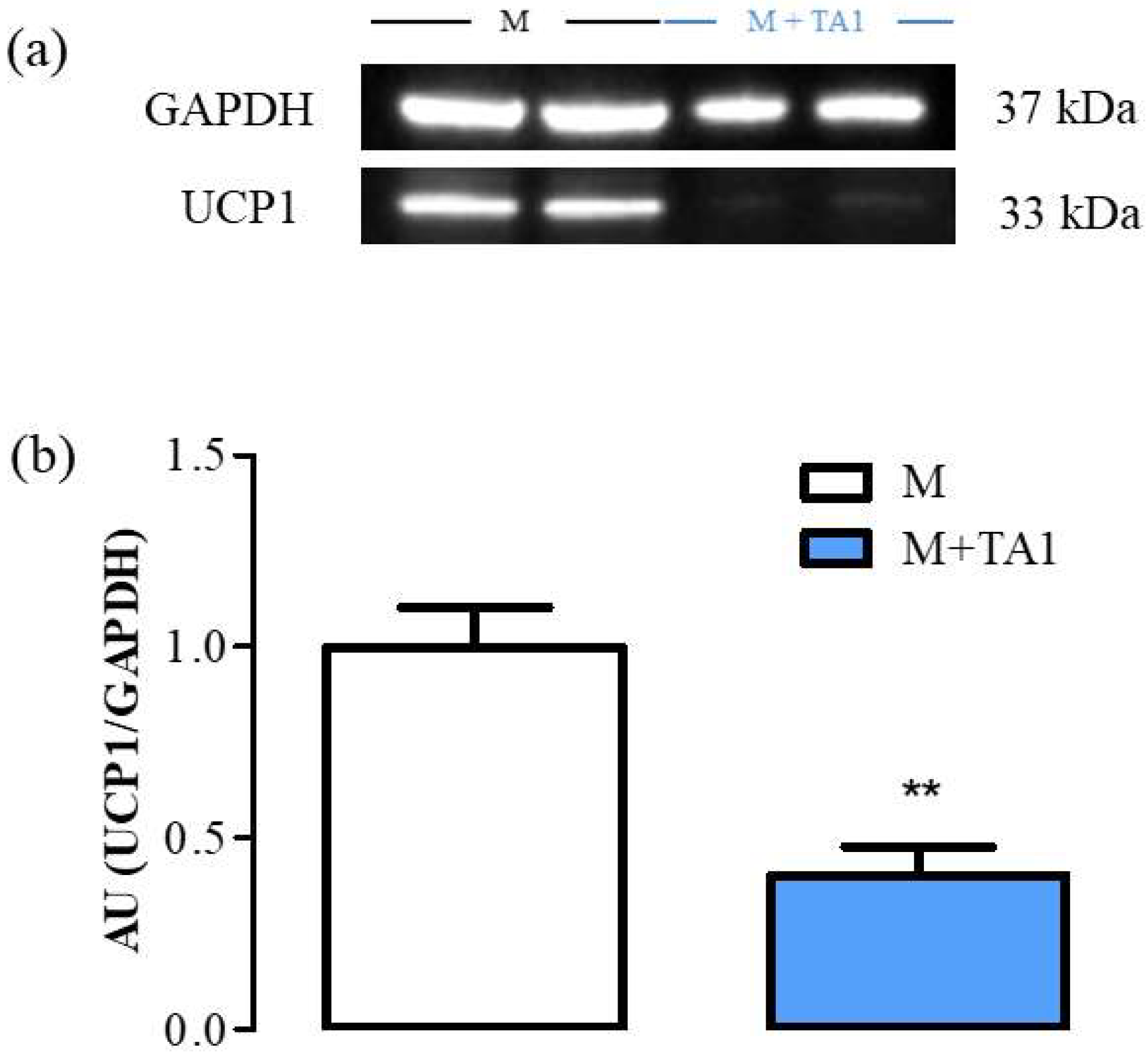
Figure 3. The levels of UCP-1 in M and M + TA1 cells. (a) Representative experiment loaded with proteins obtained for two different cell preparations. (b) Densitometric analysis is reported as the mean± standard error of the mean (SEM: n = 5 and 6 cell preparations from M and M + TA1, respectively) of arbitrary units (AU) ** p < 0.01 vs. M cells.
3.2. Evaluation of mRNA Levels for MAO (A and B) and SSAO in M + T1AM and M + TA1 Cells
The results showed that the M + T1AM cells showed lower mRNA levels for SSAO than the M cells, while both cell populations showed similar mRNA levels for MAO-A and MAO-B (Figure 3, panel a, b, ** p < 0.01). Instead, the M + TA1 cells presented significantly lower mRNA levels for SSAO, MAO-A, and MAO-B compared to the M cells (Figure 4, panel a, b, c; ** p < 0.01; *** p < 0.001).
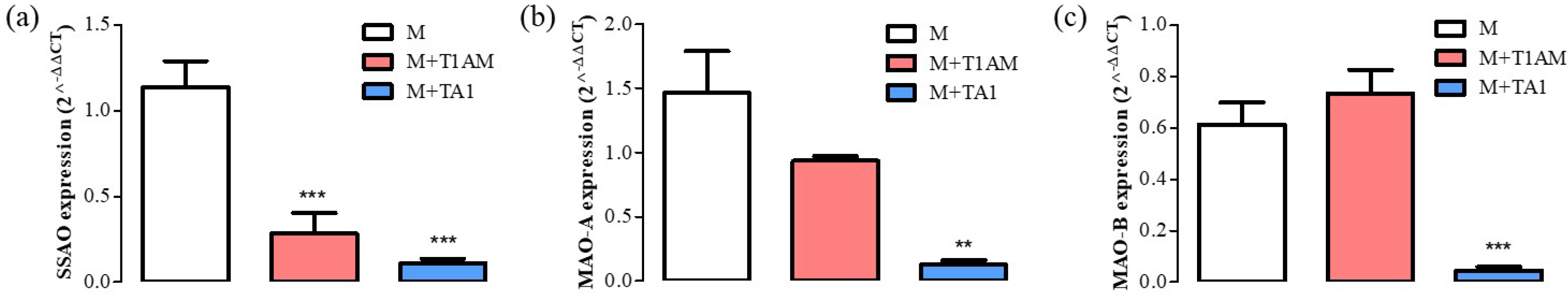
Figure 4. Differentiation marker levels in M, M + TA1, and M + T1AM cells. M, M + TA1, and M + T1AM cells. The expression levels of mRNA for (a) SSAO, (b) MAO-A, and (c) MAO-B were evaluated by RT-PCR. The mRNA expression of (a) SSAO, (b) MAO-A, and (c) MAO-B were reported as the mean± standard error of the mean (SEM) from four different cell preparations each run in triplicate: ** p < 0.01, and *** p < 0.001 vs. M cells.
3.3. MAO-B Activity
As shown above, the M + T1AM and M cells expressed mRNA levels for MAO-B at similar levels. However, it is known that the mRNA levels for MAO-B may be not in line with MAO-B enzyme activity [20]. Since MAO-B is the enzyme involved in T1AM degradation producing hydrogen peroxide and then TA1, researchers decided to evaluate MAO-B enzyme activity by using [14]C-benzylamine as substrate at conditions allowing the detection of SSAO or MAO-B respectively. As reported in Figure 5, in M + T1AM cells, SSAO activity (panel a) showed a tendency (not significant) to be lower than in M cells. Conversely, in the same cells, MAO-B activity was found to be significantly higher than in the M cells (panel b, * p < 0.05).
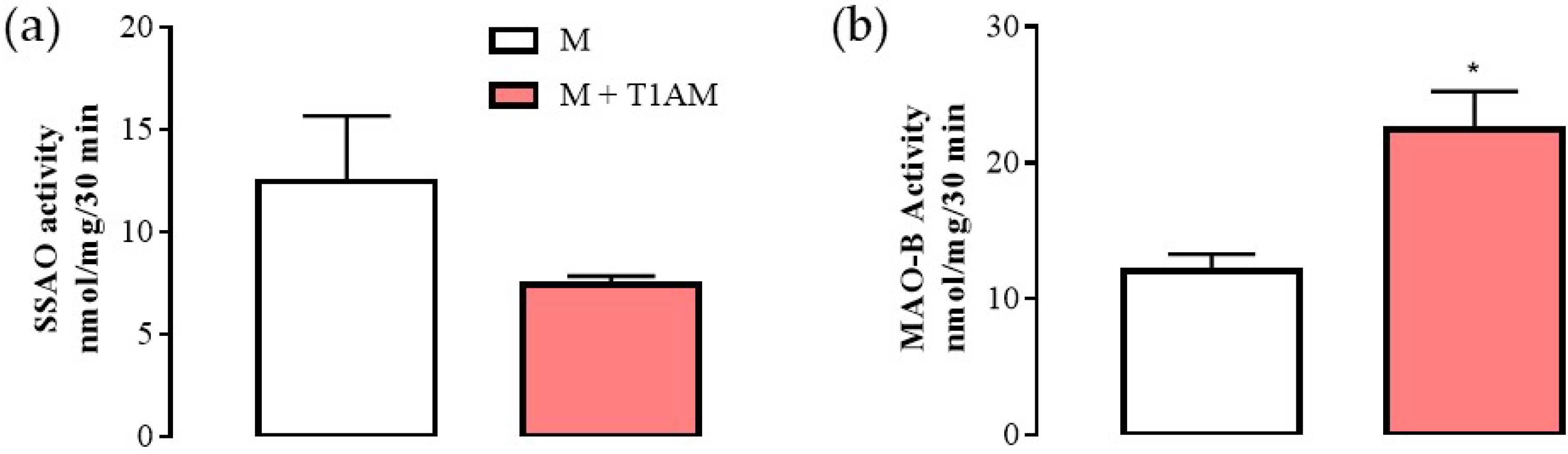
Figure 5. SSAO and MAO-B activities in M and M + T1AM cells. (a) SSAO and (b) MAO-B activities were measured radiochemically. The results are expressed as the means ± SEM of experiments (n = four different cell preparations, each run in triplicate). * p < 0.05 vs. M cells.
3.4. SIRT1 Expression and Activity in M + T1AM and M + TA1 Cells
SIRT1 is a NAD+-dependent de-acetylase involved in several cellular processes and functions, a cell redox sensor and among the positive controllers of MAO-A-promoter activity [21]. Therefore, researchers investigated the expression/activity of SIRT1-1 in the cell populations.
The results indicated that the M + T1AM and M + TA1 cells expressed SIRT1 protein levels lower and higher, respectively, than the M cells (Figure 6, Panels a, c; *** p < 0.001; Figure 6, Panels b, d; *** p < 0.001).
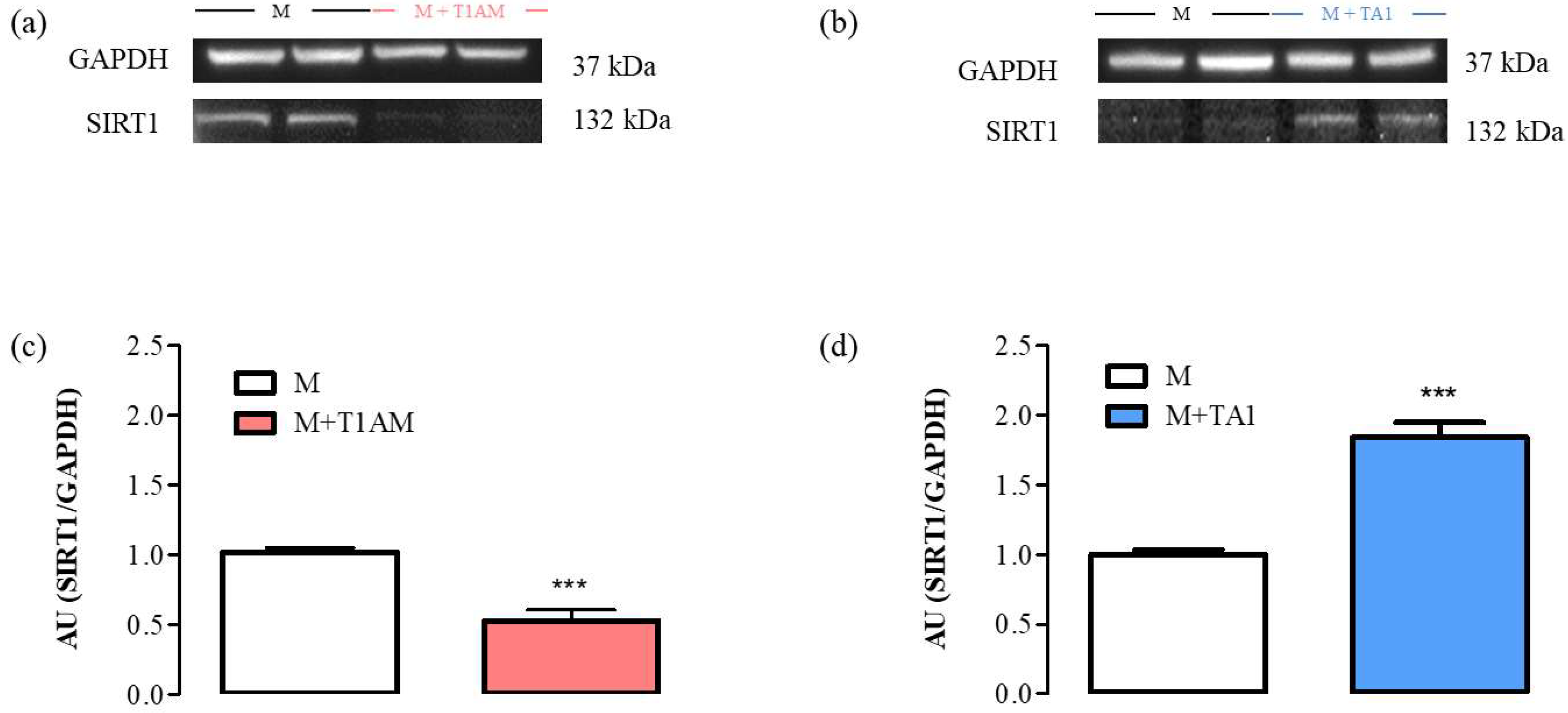
Figure 6. SIRT1 expression levels in M, M + TA1, and M + T1AM cells. M, M + TA1, and M + T1AM cells, were analyzed for the expression levels of SIRT1 by Western blot analysis. (a,b) Representative experiments are shown. Each gel was loaded with proteins obtained from two different cell preparations. (c,d) Densitometric analysis is reported as the mean± standard error of the mean (SEM: n = four cell preparations) of arbitrary units (AU. *** p < 0.001 vs. M cells.
The SIRT1 activity was then followed by measuring the expression levels of two of its substrates: H3K9ac and Acetyl-p53. According to the results, the M + T1AM and M + TA1 cells showed higher and lower H3K9ac and Acetyl-p53 protein levels, respectively, than the M cells (Figure 7, panels a, c; ** p < 0.01) (Figure 7, panels e, g; *** p < 0.001) (Figure 7, panels b, d and panels f, h; *** p < 0.001 and ** p < 0.01, respectively).
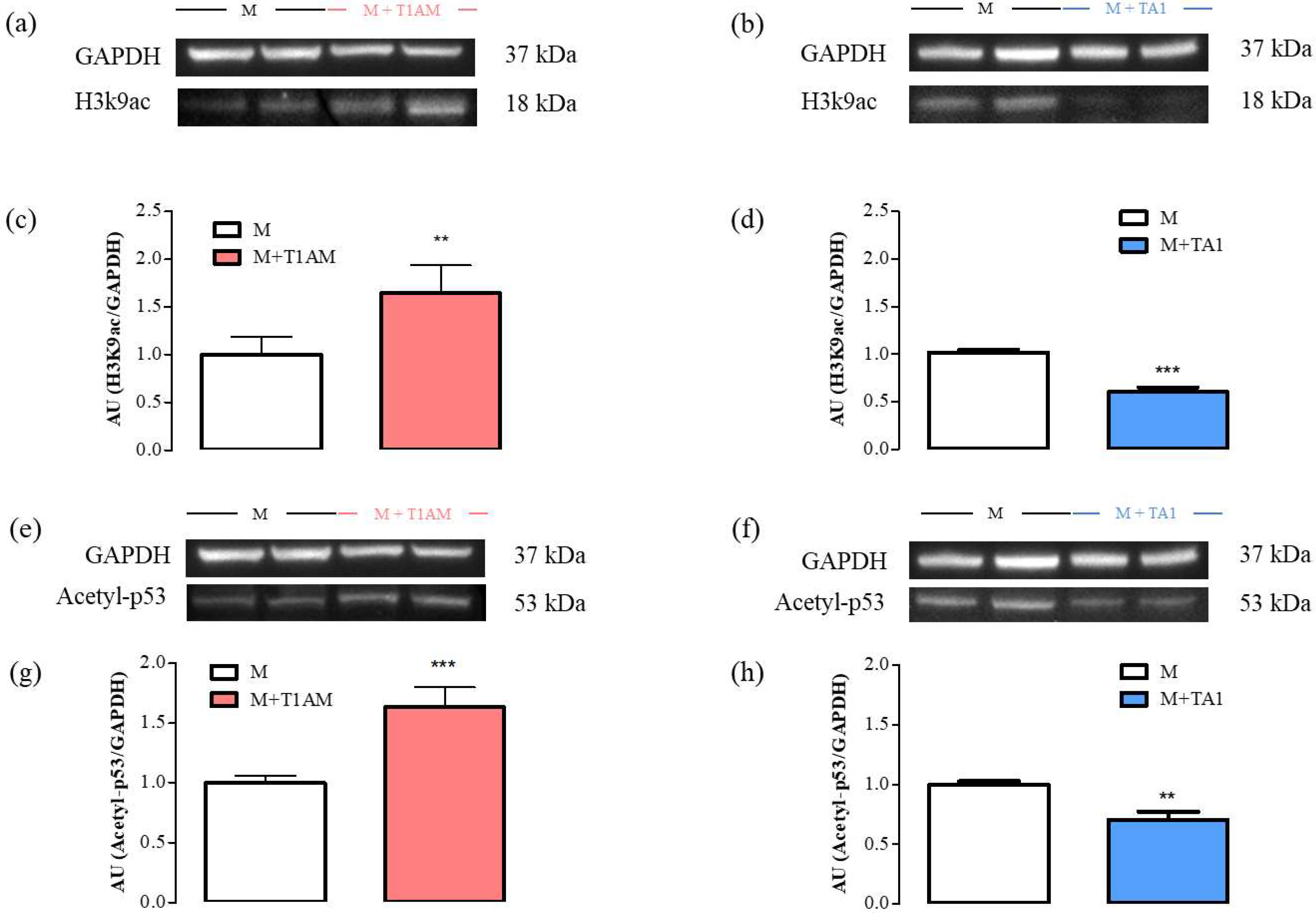
Figure 7. H3K9ac and Acetyl-p53 levels in M, M + TA1, and M + T1AM cells. M, M + TA1, and M + T1AM cells, were analyzed for their levels of H3K9ac and Acetyl-p53 by Western blot analysis. In Panels (a,b,e,f) representative experiments are shown. Each gel was loaded with proteins obtained for two different cell preparations. (c,d,g,h) Densitometric analysis is reported as the mean ± standard error of the mean (SEM: n = 4 cell preparations) of arbitrary units (AU. ** p < 0.01, *** p < 0.001 vs. M cells).
3.5. Lipid Peroxidation in M + T1AM and M + TA1 Cells
Since the M + T1AM cells acquired a pro-oxidant metabolism, including a reduction in SIRT1, researchers decided to verify whether the cells showed signs of ROS-dependent lipid damage, including lipoperoxidation. Consistently with the reduction in SIRT1 levels and activity, M + T1AM cells showed significantly higher TBARS levels than the M cells (Figure 8; * p < 0.05). Notably, the TBARS levels were similar in the M and M + TA1 cells.
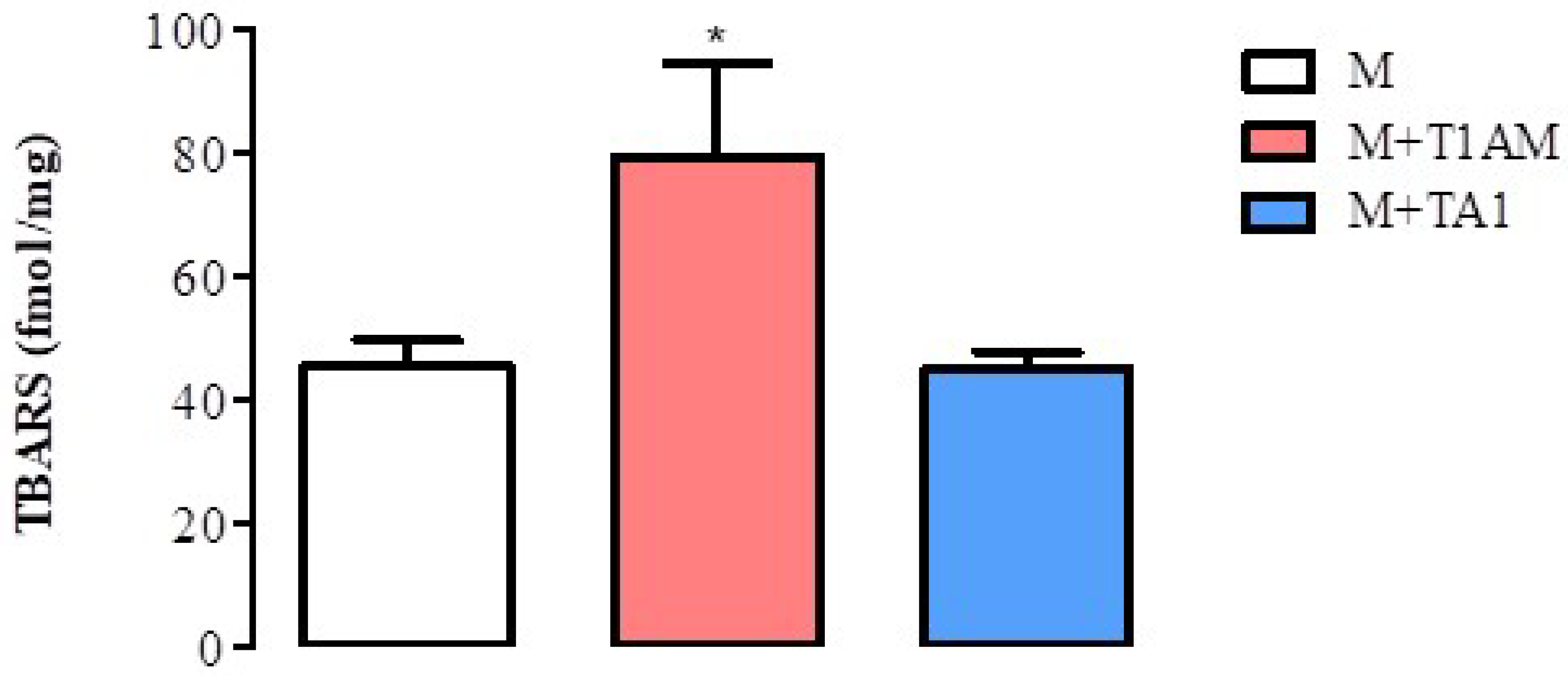
Figure 8. TBARS levels in M, M + TA1, and M + T1AM cells. M, M + TA1, and M + T1AM cells were analyzed for TBARS levels. The results are expressed as the mean± standard error of the mean (SEM) from four different cell preparations, each run in triplicate. * p < 0.05 vs. M cells.
This entry is adapted from the peer-reviewed paper 10.3390/ijms23052718
References
- Scanlan, T.S.; Suchland, K.L.; Hart, M.E.; Chiellini, G.; Huang, Y.; Kruzich, P.J.; Frascarelli, S.; Crossley, D.A.; Bunzow, J.R.; Ronca-Testoni, S.; et al. 3-Iodothyronamine is an endogenous and rapid-acting derivative of thyroid hormone. Nat. Med. 2004, 10, 638–642.
- Köhrle, J.; Biebermann, H. 3-Iodothyronamine-A Thyroid Hormone Metabolite with Distinct Target Profiles and Mode of Action. Endocr. Rev. 2019, 40, 602–630.
- Laurino, A.; Gencarelli, M.; Raimondi, L. The 3-iodothyronamine (T1AM) and the 3-iodothyroacetic acid (TA1) indicate a novel connection with the histamine system for neuroprotection. Eur. J. Pharmacol. 2021, 912, 174606.
- Duicu, O.M.; Lighezan, R.; Sturza, A.; Balica, R.; Vaduva, A.; Feier, H.; Gaspar, M.; Ionac, A.; Noveanu, L.; Borza, C.; et al. Assessment of Mitochondrial Dysfunction and Monoamine Oxidase Contribution to Oxidative Stress in Human Diabetic Hearts. Oxid. Med. Cell. Longev. 2016, 2016, 8470394.
- Bianchi, P.; Kunduzova, O.; Masini, E.; Cambon, C.; Bani, D.; Raimondi, L.; Seguelas, M.H.; Nistri, S.; Colucci, W.; Leducq, N.; et al. Oxidative stress by monoamine oxidase mediates receptor-independent cardiomyocyte apoptosis by serotonin and postischemic myocardial injury. Circulation 2005, 112, 3297–3305.
- Yin, L.; Li, J.; Wang, J.; Pu, T.; Wei, J.; Li, Q.; Wu, B.J. MAOA promotes prostate cancer cell perineural invasion through SEMA3C/PlexinA2/NRP1-cMET signaling. Oncogene 2021, 40, 1362–1374.
- Accorroni, A.; Rutigliano, G.; Sabatini, M.; Frascarelli, S.; Borsò, M.; Novelli, E.; Bandini, L.; Ghelardoni, S.; Saba, A.; Zucchi, R.; et al. Exogenous 3-Iodothyronamine Rescues the Entorhinal Cortex from β-Amyloid Toxicity. Thyroid 2020, 30, 147–160.
- Landucci, E.; Gencarelli, M.; Mazzantini, C.; Laurino, A.; Pellegrini-Giampietro, D.E.; Raimondi, L. N-(3-Ethoxy-phenyl)-4-pyrrolidin-1-yl-3-trifluoromethyl-benzamide (EPPTB) prevents 3-iodothyronamine (T1AM)-induced neuroprotection against kainic acid toxicity. Neurochem. Int. 2019, 129, 104460.
- Doyle, K.P.; Suchland, K.L.; Ciesielski, T.M.; Lessov, N.S.; Grandy, D.K.; Scanlan, T.S.; Stenzel-Poore, M.P. Novel thyroxine derivatives, thyronamine and 3-iodothyronamine, induce transient hypothermia and marked neuroprotection against stroke injury. Stroke 2007, 38, 2569–2576.
- Zhou, H.; Hu, B.; Liu, X. Thyroid Hormone Metabolite 3-Iodothyronamine (T1AM) Alleviates Hypoxia/Reoxygenation-Induced Cardiac Myocyte Apoptosis via Akt/FoxO1 Pathway. Med. Sci. Monit. 2020, 26, e923195.
- Ghanian, Z.; Maleki, S.; Reiland, H.; Bütz, D.E.; Chiellini, G.; Assadi-Porter, F.M.; Ranji, M. Optical imaging of mitochondrial redox state in rodent models with 3-iodothyronamine. Exp. Biol. Med. 2014, 239, 151–158, Erratum in Exp. Biol. Med. 2014, 239, 770.
- Sacripanti, G.; Lorenzini, L.; Bandini, L.; Frascarelli, S.; Zucchi, R.; Ghelardoni, S. 3-Iodothyronamine and 3,5,3′-triiodo-L-thyronine reduce SIRT1 protein expression in the HepG2 cell line. Horm. Mol. Biol. Clin. Investig. 2020, 41, 20190045.
- Assadi-Porter, F.M.; Reiland, H.; Sabatini, M.; Lorenzini, L.; Carnicelli, V.; Rogowski, M.; Selen Alpergin, E.S.; Tonelli, M.; Ghelardoni, S.; Saba, A.; et al. Metabolic Reprogramming by 3-Iodothyronamine (T1AM): A New Perspective to Reverse Obesity through Co-Regulation of Sirtuin 4 and 6 Expression. Int. J. Mol. Sci. 2018, 19, 1535.
- Santos, L.; Escande, C.; Denicola, A. Potential Modulation of Sirtuins by Oxidative Stress. Oxid. Med. Cell. Longev. 2016, 2016, 9831825.
- Chen, Y.; Zhang, H.; Ji, S.; Jia, P.; Chen, Y.; Li, Y.; Wang, T. Resveratrol and its derivative pterostilbene attenuate oxidative stress-induced intestinal injury by improving mitochondrial redox homeostasis and function via SIRT1 signaling. Free Radic. Biol. Med. 2021, 177, 1–14.
- Wang, L.; Li, Q.; Yan, H.; Jiao, G.; Wang, H.; Chi, H.; Zhou, H.; Chen, L.; Shan, Y.; Chen, Y. Resveratrol Protects Osteoblasts against Dexamethasone-Induced Cytotoxicity through Activation of AMP-Activated Protein Kinase. Drug Des. Dev. Ther. 2020, 14, 4451–4463.
- Gencarelli, M.; Laurino, A.; Landucci, E.; Buonvicino, D.; Mazzantini, C.; Chiellini, G.; Raimondi, L. 3-Iodothyronamine Affects Thermogenic Substrates’ Mobilization in Brown Adipocytes. Biology 2020, 9, 95.
- Xu, D.F.; Liu, Y.J.; Mao, Y.F.; Wang, Y.; Xu, C.F.; Zhu, X.Y.; Jiang, L. Elevated angiotensin II induces platelet apoptosis through promoting oxidative stress in an AT1R-dependent manner during sepsis. J. Cell. Mol. Med. 2021, 25, 4124–4135.
- Cui, Y.; Liu, K.W.; Liang, Y.; Ip, M.S.; Mak, J.C. Inhibition of monoamine oxidase-B by selegiline reduces cigarette smoke-induced oxidative stress and inflammation in airway epithelial cells. Toxicol. Lett. 2017, 268, 44–50.
- Ramsay, R.R. Molecular aspects of monoamine oxidase B. Prog. Neuropsychopharmacol. Biol. Psychiatry 2016, 69, 81–89.
- Libert, S.; Pointer, K.; Bell, E.L.; Das, A.; Cohen, D.E.; Asara, J.M.; Kapur, K.; Bergmann, S.; Preisig, M.; Otowa, T.; et al. SIRT1 activates MAO-A in the brain to mediate anxiety and exploratory drive. Cell 2011, 147, 1459–1472.
This entry is offline, you can click here to edit this entry!
