Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Food Science & Technology
|
Chemistry, Analytical
The pan-European distributed Research Infrastructure for Promoting Metrology in Food and Nutrition (METROFOOD-RI) has evolved in the frame of the European Strategy Forum on Research Infrastructures (ESFRI) to promote high-quality metrology services across the food chain. The METROFOOD-RI comprises physical facilities and electronic facilities. The former includes Reference Material plants and analytical laboratories (the ‘Metro’ side) and also experimental fields/farms, processing/storage plants and kitchen-labs (the ‘Food’ side).
- European research infrastructures
- METROFOOD-RI
- food metrology
- agrifood
- food authenticity
- food safety
- nutrition
- One Health
- reference materials
1. Research Infrastructures in Europe–METROFOOD-RI Evolution
Since 2002, the European Strategy Forum on Research Infrastructures (ESFRI) is the strategic instrument of the European Council (EC) “to support a coherent and strategy-led approach to policymaking on research infrastructures in Europe” [1]. As a result, facilities, resources, or services of a unique nature are identified by the European research communities as potent Research Infrastructures (RIs) to conduct and support top-level research activities in their domains. ESFRI selects proposals of strategic importance for the European Research Area (ERA), with excellent scientific case and an adequate level of maturity to become ESFRI Projects so that they can support their timely implementation as new or updates of RIs within a ten-year term. The successfully implemented RIs may gradually become ESFRI Landmarks. The latter are considered important elements of competitiveness of the ERA because they have proven capability of delivering science services and granting user access. RIs and Landmarks are expected to be optimally managed according to well-designed governance plans and legally supported by participating Member States [2]. Over the past decade, multilateral agreements for building up RIs appeared in all fields of science and technology, and several of them are already organised as European Research Infrastructure Consortia (ERIC) to fulfil the legal status requirements for their operation at national, European and international level.
The agrifood sector represents one of the largest and most important socio-economic sectors worldwide and also within the European Union (EU). The sector faces great challenges as practices prevailing for more than a century do not guarantee its resilience and fulfilment of the ultimate goal, i.e., “to feed the world”. Food security for all people at all times is currently challenged, not only because of global population growth, but also due to climate crisis, increasing poverty, and uncertainty that causes vast numbers of people to migrate at an uncontrolled pace. The consequences of the advancing COVID-19 pandemic have underscored the need for global cooperation in the sector. The serious disruption in the food chain that was experienced during the lockdown period had multiple negative effects, which are currently augmented by the energy crisis that hit primary production, manufacture, and trade of food products. The four pillars of food security, i.e., availability, access, utilisation, and stability were compromised worldwide, including Europe. Nevertheless, scientific and technological progress in the sector is continuous and all interested parties are eager to address current challenges in an effective and innovative way in accordance with global and EU initiatives and strategies. Priorities are set globally to promote sustainability of the sector with a focus on increasing agricultural yields and efficiency; limiting environmental burden on biodiversity, soils, water and air; reducing food losses and waste; and promoting patterns for healthier and less resource-intensive diets.
Following the deployment of the 17 Sustainable Development Goals (SDGs) by the United Nations General Assembly in 2015 (UN 2030 Agenda), many political initiatives at the global and EU level were put into force to implement the UN 2030 Agenda. Given that at least eight of the SDGs are directly or indirectly connected to agriculture, reliable and traceable measurements are crucial in supporting economic competitiveness, manufacturing and trade by ensuring traceability and sustainability of the agrifood systems in a circular economy prospect [3]. In this context, the pan-European METROFOOD-RI Infrastructure for Promoting Metrology in Food and Nutrition [4] was evolved to promote high-quality metrology services along the whole food chain, i.e., ’from farm to fork’, through FAIR (findable, accessible, interoperable, re-usable) data management practices. FAIR data have already been recognised as of strategic importance for the ERA [5].
Currently, METROFOOD-RI runs its preparatory phase (PP), which is financed under the EU-Horizon 2020 project METROFOOD-PP (GA No 871083) to support its activities for self-assembly as a new distributed RI in the food and nutrition domain. It combines a physical and an electronic component with multidisciplinary facilities that are distributed across 18 European countries and can provide scientific services in an integrated and collaborative way (Figure 1).
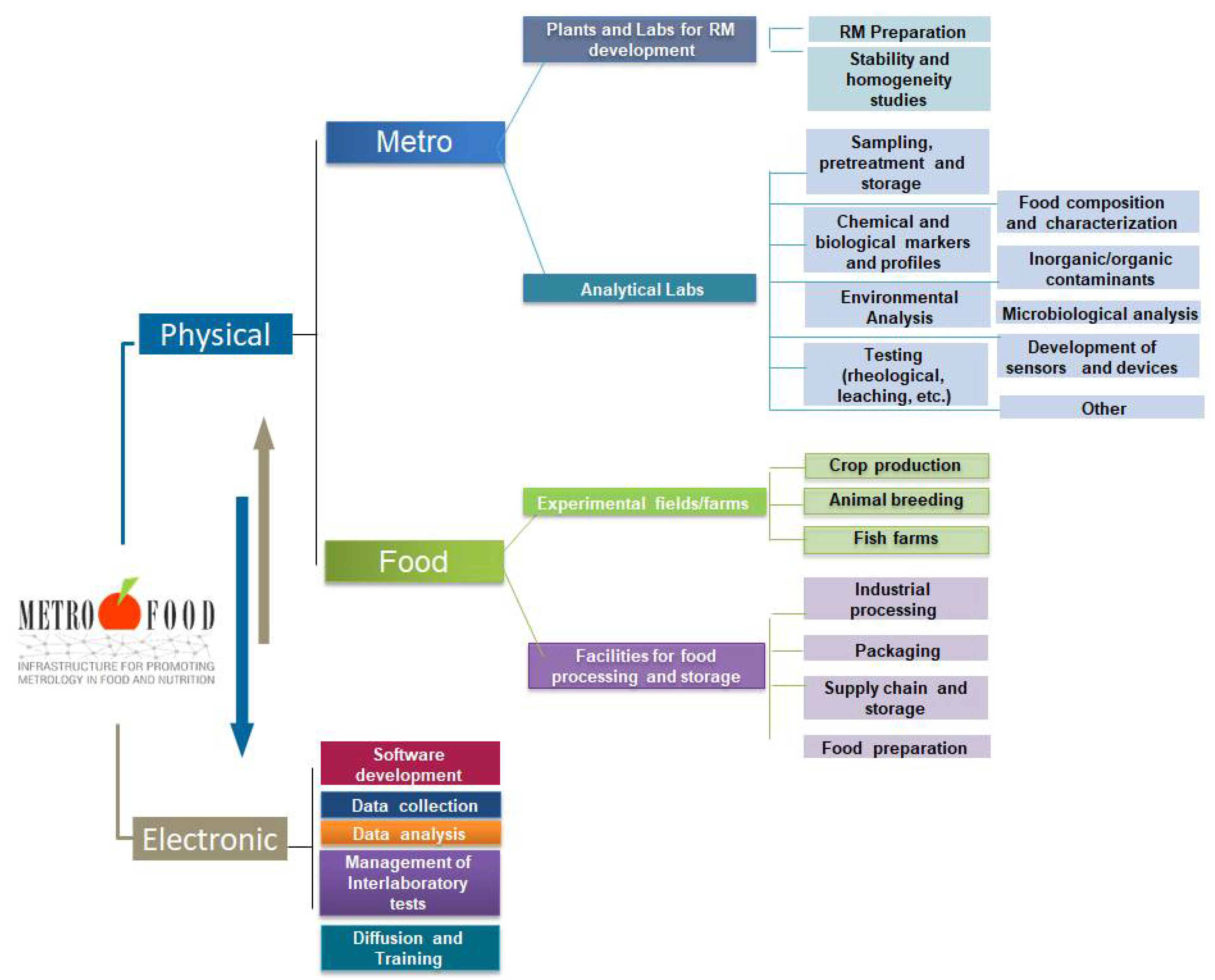
Figure 1. Basic service and operation components of the METROFOOD-RI. The Physical-RI consists of two sides, the ‘Metro’ and ‘Food’ ones that are integrated and coordinated by the Electronic-RI.
The physical component of the infrastructure has two sides: one consisting of Reference Material plants and analytical laboratories for the development and validation of new Reference Materials and new methods (the ‘Metro’ side), and another one consisting of experimental fields/farms for crop production/animal breeding, plants for food processing and storage, and kitchen-labs for food preparation (the ‘Food’ side). All physical facilities are integrated and coordinated by the electronic component of the infrastructure. The latter will provide an access platform to share and integrate knowledge and data on metrological tools for food analysis, focusing on food composition, nutritional value, safety issues, and authenticity markers. The METROFOOD-RI is well positioned in the ESFRI landscape and builds a strong network of facilities that are pertinent with many other domains, RIs and networks. Strengthening these links with joint strategies and complementary activities in a long-term schedule through the infrastructure operation requires a consolidated scientific plan of services in the domain. The development and continuous updating of a scientific plan is a core activity essential both for identifying strengths and weaknesses of the RI in relation to emerging topics of scientific research in food and nutrition, but also for aligning them with wider societal goals and challenges. The offer is addressed to a broad set of users, such as researchers and public and private laboratories; food business operators and producer associations; policy makers and food inspection and control agencies; and consumer associations and citizens [5].
2. Key Thematic Areas in the METROFOOD-RI Scientific Plan
The strategic priorities for Reference Materials and the major components of food integrity are illustrated in Figure 2 and discussed in the following subsections. General concepts, METROFOOD-RI capacity and perspectives are presented for each of the thematic areas.
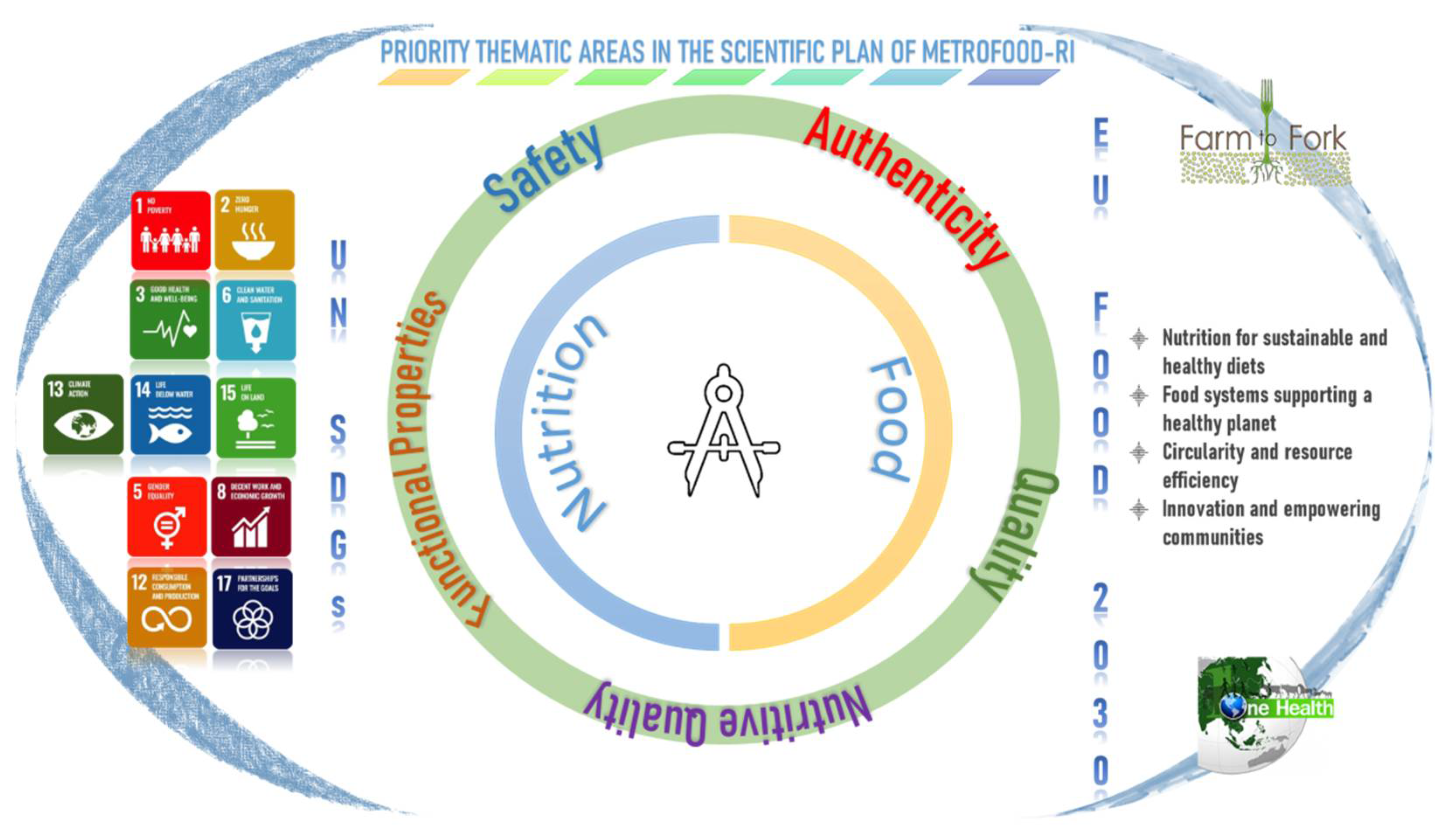
Figure 2. Key thematic areas covered in the METROFOOD-RI scientific plan and relevance to the global and EU policy framework in the agrifood sector.
2.1. Reference Materials
The production of Reference Materials (RMs) is a key activity for the improvement and maintenance of a worldwide coherent measurement system. RM is a generic term that includes Certified Reference Materials (CRMs), matrix-RMs, primary or secondary measurement standards, calibrants (pure substances for calibration), and quality control materials [6]. METROFOOD-RI, aiming at supporting metrology in food and nutrition, provides as one of its core services the development and production of new RMs, which should be exploited to cover the aforementioned identified needs.
2.1.1. Capacity Building in Production
The available facilities for RM production, along with the further capacity expansion planned with upgrading, allow METROFOOD-RI to promote the development of new—even customised—matrix-(C)RMs covering new matrix/analyte combinations suitable to fulfil emerging analytical requirements (e.g., stable isotope analysis), as well as R&D activities on innovative RMs (e.g., multipurpose, single-use, double-phase, driven), and their production at both a small and a large (industrial) scale [9]. The recently launched application RM-App as free e-service of the infrastructure (https://www.metrofood.eu/access/e-services.html) (accessed on 19 November 2021) is an open access tool supporting the search for ‘fit for the purpose’ RMs of interest specifically for the agrifood sector, covering the worldwide production of matrix-RMs and pure substances for calibration. Moreover, it helps to identify gaps for better planning and directing of future research activities of the infrastructure. Table 1 presents some examples of emerging topics in this area that were prioritised in line with the key points of the Strategic Research Agenda for Metrology in Europe, defined by EURAMET [3], and suggestions made by the European Commission’s Joint Research Centre (JRC) [10].
Table 1. Identified gaps in the field of Reference Material (RM) design and production and emerging topics that are of high priority for the METROFOOD-RI consortium.
| Reference | Identified Gaps and Emerging Topics |
|---|---|
| [11] | traceable measurements of residues and pathogens (viruses, bacteria, toxins) |
| RMs for species identification, embedded nanoparticles microplastics, allergens | |
| [12] | additional (C)RMs in the less-populated sectors of the protein-fat-carbohydrate (P/F/C) AOAC (Association of Official Analytical Collaboration, International) triangle |
| [13] | RMs with assigned values for vitamin D and its metabolites, vitamin K and folate vitamins |
| RMs for arsenic parameters, emerging contaminants, persistent organic pollutants in marine biota, GMOs, alkaloids, mineral oil hydrocarbons, glyphosate in cereals (for which there is a controversy on Maximum Residue Level), food contact materials migration, cocoa | |
| [13][14] | RMs for acrylamide in other than infant formula products |
| [15] | RMs for sensory analyses, panel tests, authenticity (markers/profiles), identity or other qualitative properties |
Marketing needs for the declaration of geographical origin or a property in order to differentiate a product (e.g., health claims for olive oils—Polar phenols) can be the driving force for the development of RMs for identity or qualitative properties. The envisaged development of such RMs is expected to open new opportunities in the field of traceability and authenticity. At the same time, the metrological basis for setting the foundations of the estimation of uncertainty in the evaluation of nominal properties has already been laid down [15]. This approach will therefore allow the opportunity for producing RMs for sensory and texture analysis.
2.1.2. Capacity Building in Characterisation
Characterisation is one of the steps in the production process of RMs that requires measurement of values for a variety of attributes and where calculation of uncertainties is necessary (e.g., for certified or reference values). RM characterisation requires minimally biased measurement procedures with low uncertainty as results will then be combined with those from homogeneity and stability assessment. Characterisation steps according to the recently revised ISO/Guide 35:2017 [16] can be achieved using (a) a single (primary) method in a single laboratory; (b) two or more independent reference methods in one or several laboratories; (c) one or more methods of demonstrable accuracy, performed by a network of competent laboratories; or (d) an approach providing method-specific, operationally defined property values, using a network of competent laboratories. It is evident that validated methods should be preferred when available. The involved laboratories should provide evidence of competence for the measurand in question independent of the measurements on the candidate CRM. Τwo further points should be taken into account. The first one, raised by the IAEA (International Atomic Energy Agency) regards the inclusion of nuclear methods such as neutron activation analysis (NAA) for the characterisation of RMs when appropriate for the venture. NAA performance is considered as equal to that of primary methods recognised by the Consultative Committee for Amount of Substance: Metrology in Chemistry and Biology (CCQM) [17]. In addition, it must be considered that NAA is one of the most suitable methods for performing homogeneity studies. The second point is the use of ID (isotope dilution)-LC-MS and ID-LC-MS/MS and also ID-GC-MS and ID-GC-MS/MS methods, which provide more accurately assigned values of lower expanded uncertainties, potentially < 3% depending on material homogeneity from a metrological point of view, to assign values for vitamins in supplements and foods instead of microbiological assays wherever applicable [12].
In the field of pathogens and GMO detection, digital PCR (dPCR) is recommended as a candidate reference method for the characterisation of DNA/RNA RMs, due to the precise quantification without the need for a reference standard [18]. Homogeneity and stability studies (under thermal and luminous stress) are also essential in order to properly develop RMs.
2.1.3. Perspective
The added value of METROFOOD-RI is that its physical infrastructure allows to combine the plants for RM development and production, with the whole spectrum of analytical laboratories that can be used for any type of chemical, physicochemical, and microbiological characterisation, even for the development of new multipurpose-Reference Materials, as well as for homogeneity studies. Furthermore, the ‘Food’ side physical facilities allow the in-house preparation of raw materials of well-known origin, which can be used to prepare new RMs, e.g., for authenticity studies (to validate geographical, botanical or zoological origin claims), or to develop driven-RMs, i.e., RMs naturally spiked directly in the field or in plants, thus much more representative of the naturally occurring contamination. As an example, METROFOOD-RI partners, together with other national and international institutes, recently participated in an inter-laboratory assessment (CCQM-K140) of stable carbon isotope ratio determination of bulk honey [19]. Finally, the integration with the e-component enables statistical analysis, data processing, organisation, and management of proficiency testing (PT).
2.2. Food Authenticity and Traceability
2.2.1. Toward an Anti-Fraud Scientific Alliance
Food purity and authenticity is becoming an uprising issue for food authorities worldwide as it has been in the beginning of the 20th century because illegal practices can lead to severe health consequences for consumers and disrupt trust and fair trade. To update this part of the scientific plan of the METROFOOD-RI in view of the ERIC status, policy views, initiatives, and research trends along with analytical breakthroughs on authenticity testing were taken into account. A literature search pointed out that important global and European initiatives to combat food fraud and sustain fair trade were established in recent years. Amendments to legislation in the U.S. and EU, Think Tanks and consumer fora for discussions and proposals, and collaboration between Europol and Interpol aim at combating the problem effectively. Special mention should be given to the AOAC International Standards and methods development program [21] that included actions on food authenticity methods covering targeted and non-targeted approaches in an effort to establish Standard Method Performance Requirements and adoption of single-laboratory or multi-laboratory validated methods. Such actions shape the metrology culture in the field of food authentication and highlight the importance of the complementary expertise existing among the METROFOOD-RI partners. The provision of high-quality analytical and metrological services for food authenticity testing will also require joint actions with other relevant networks in an open science environment. At a European level it is of utmost importance to connect with and support the activities of the recently established Knowledge Centre for Food Fraud and Quality (KCFFQ) [22] created by the EU Food Fraud Network and operated by the European Commission’s JRC. Participating EU Member State authorities [23][24]. have prioritised several issues for action against food fraud, some of which are fully aligned with the key objectives of the METROFOOD-RI from a scientific perspective. The creation of centres for analytical competence, harmonisation of analytical methods and creation of open access compositional databases for food commodities are among these top priority actions.
2.2.2. State-of-the-Art Analytical Tools
A bibliometric search in the Web of Science database (accessed on 9 October 2021) using the keyword topic ‘food authentication’ signified that the field became extremely popular to scientific researchers since 2015, as the number of relevant publications has grown almost exponentially with regard to the previous decade. Thus, on average 300–500 research articles about novel analytical methods and food authentication techniques were published annually during this period. It is noteworthy that the number of review articles published in the abovementioned period about strengths and weaknesses of the applied protocols, food-matrix-related limitations and other relevant trends, and concepts and considerations was also very high (290 hits); 80% of these articles were published after 2018. The most cited articles in the field gather comprehensive information about data processing methods (e.g., chemometrics, multivariate modelling, data fusion) [25][26][27], blockchain technology for data storage [28], electronic sensors for in-line monitoring [29], non-targeted fingerprinting approaches [30], and DNA-based methods [31], and offer overviews of applications per food category [32] or analytical technology breakthroughs [33]. Despite the great wealth of information about the ‘proof of concept’ of the employed methodologies, the quality and comparability of the results that are critical for the transfer and application of the method into real food control systems are rarely discussed. The terms ‘metrology’, ’harmonisation’ or ’standardisation’ appear scarcely in the relevant publications. Some terms that are more specific with the evaluation of an analytical method performance such as ‘validation’ and ‘robustness’ are addressed in very few of those papers [27][30][34]. Among the analytical tools that are commonly used in this research field, those based on chromatographic and spectroscopic techniques prevail. An overview of the basic principles and relevant instrumentation is given in Table 2.
Table 2. Overview of analytical techniques commonly used in authenticity and traceability studies [33][34][35][36][37][38][39][40][41][42][43][44].
| Analytical Technique | Principle | Variation | |
|---|---|---|---|
| Chromatographic | |||
| (Ultra) High performance liquid chromatography (U)HPLC | Adsorption and/or partition of target analytes between mobile (liquid or gas) and stationary phase | Separation | Hyphenation to spectrometry MS/MS TOF Triple quadruple |
| Gas chromatography (GC) | |||
| Multidimensional chromatography (LC x LC, GC x GC, GC x LC, LC x GC) | |||
| Spectroscopic | |||
| Infrared | Absorption of electromagnetic radiation | Vibration of bonds of molecular functional groups (prerequisite change of dipole moment) | Fourier-Transform-Mid-infrared (FT-MIR) Fourier-Transform-Near-infrared (FT-NIR) |
| Raman | Vibration of bonds of molecular functional groups (prerequisite change of polarizability) | Raman Fourier-Transform-Raman (FT-Raman) |
|
| Ultraviolet–visible (UV–Vis) | Excitation of electrons | ||
| Fluorescence | Energy emission after atom excitation to higher energy levels | Synchronous (SyF) Front phase (FP) |
|
| Nuclear magnetic resonance (NMR) | Absorption of radiofrequency radiation by atomic nuclei with non-zero spins | Resonance | High-resolution NMR Low-resolution NMR Liquid/solid-state |
| X-ray | Absorption and scattering of X-ray beams | Image | X-ray fluorescence (XRF) |
| Mass spectrometry | Formation of ions with different mass-to-charge ratio | Separation in an electromagnetic field | Isotope Ratio (IR-MS), Inductively Coupled Plasma (ICP-MS) Thermal Ionisation (TI-MS) Proton transfer reaction (PTR-MS) Matrix-assisted laser desorption/ionisation Time-of-Flight (MALDI-TOF) Direct Analysis in Real Time (DART) Liquid Extraction Surface Analysis (LESA) |
| Molecular | |||
| Polymerase chain reaction (PCR) | Amplification of DNA fragments | Separation of DNA fragment sizes by gel-electrophoresis (sequencing), melt curves of DNA fragments |
DNA barcoding high-resolution melting (Bar-HRM) Droplet digital PCR (ddPCR), High-resolution melting (HRM), Loop-mediated isothermal amplification (LAMP), Next-generation sequencing (NGS) Polymerase chain reaction (PCR) Real-time quantitative PCR (qPCR), Restriction-fragment-length polymorphism (RFLP) PCR Single-Strand Conformation Polymorphisms (PCR-SSCP) Random amplified polymorphic DNA (RAPD), Peptide Nucleic Acid (PNA) DNA fingerprinting |
| Immunological | |||
| Ligand binding (LB) | Complex formation between antigen (target protein) and antibody | Production of a detectable signal (usually colour) | Enzyme-linked immunosorbent assay (ELISA) |
2.2.3. Perspective
Currently, there is an array of conventional and emerging analytical methods laying on different principles and instrumentation. Literature findings signify that there is still a lot of work to be completed before various analytical protocols become harmonised, legally robust or commercially relevant. To this direction, METROFOOD-RI will plan research and implement collaborative actions to promote metrology in food authentication and traceability applications. The consortium aims at providing consulting, training and analytical solutions to any interested parties (e.g., national authorities, industry).
It is generally accepted that food authentication workflows involve careful selection of representative and authentic reference samples, suitable analytical methods, reliable and unbiased chemometric analysis and unambiguous metabolite identification. The targeted approach, aiming at determining known molecules (e.g., the adulterant), is suggested when a suspect product or fraud incident needs to be confirmed and is very useful to support authenticity assurance to food manufacturers or to ensure the integrity of the food supply chain. The non-targeted approach requires the use of valid reference data sets and provides screening capability to safeguard that very few incidents may evade detection. Implementation of such methods into routine analysis and food surveillance requires thorough validation [36]. Consideration of current legislation gaps and communication with all interested parties is of utmost importance for the infrastructure in order for its scientific plan to be effective in this thematic area.
2.3. Food Safety
2.3.1. A matter of Societal Importance
The legislative frame of the EU considers in a holistic way the safety of food, feed, animal well-being, plant protection and the environment in its General Food Law since 2000 when the European Food Safety Authority (EFSA) was established as the central authority to provide scientific support to the European Commission and the Parliament. Therefore, subsequent regulations, all types of documents, and current policies were the starting point for updating the safety component of the scientific plan of METROFOOD-RI from farm to fork.
Starting from the farm and taking into consideration the recent report from the EC for statistics on pesticides, it is acknowledged that “pesticides are a cause of pollution and have a direct effect especially on the state of biodiversity, water bodies, and soils. To ensure that these impacts are addressed appropriately, it is essential that policy makers are able to quantify the risk and the level of pesticide pollution” [45]. It is also acknowledged that, “The EU framework aims to achieve sustainable use of plant protection products (PPPs) by reducing risks and impacts on human health and environment and promoting integrated pest management”[46]. Both the EU Commission and Member States have taken action to promote the sustainable use of PPPs, but there has been limited progress in measuring and reducing the associated risks. Consequently, it is mandatory to develop eco-sustainable alternative tools that might be of inclusion into the low risk [47] or basic substances (Article 23) catalogues.
In the following paragraphs, major sources of hazards along with current analytical gaps and challenges that METROFOOD-RI scientific plan prioritises are discussed.
2.3.2. Capacity Building in Assessing Chemical Hazards
Chemicals in food are considered globally as a top safety issue and are of concern in international trade transactions [48]. Contaminants [49] are substances that are not intentionally present in food. They may be found in foods as a consequence of the various steps of its production, packaging, transport and storage. Additionally, new toxic residues in food are detected due to the application of new industrial processes, agricultural activities, and environmental pollution. Contaminants of emerging concern or emerging contaminants are compounds with either no defined maximum levels in the EU legislation yet, or having maximum levels, which need to be revised [52] because of new data availability or the development of new analytical tools. A literature search for the 2015-onward timespan indicates the prevalence of perfluorinated compounds (PFCs), polybrominated biphenyls (PBBs), nanomaterials, marine biotoxins such as palitoxins and spirolides, and the new generation of pesticides, antibiotics and coccidiostats. Emerging mycotoxins (enniatins, beauvericin, moniliformin, fusaproliferin, fusaric acid, culmorin, utanolide, sterigmatocystin, emodin, mycophenolic acid, alternariol, alternariol monomethyl ether, and tenuazonic acid) have also been recently proved to be present in agrifoods and feeds, exerting serious toxicity effects. Antibiotics, persistent organic pollutants (POPs) namely, PBDEs, PCBs, OCs, PAHs, and OPs, perfluoroalkyl substances (PFASs) and parabens (endocrine disruptors) are of increasing concern. Lately, there is growing concern about the presence of microplastics in the environment and their subsequent transfer in the food chain [53][54].
Lack of methods of analysis or methods with poor performance that are in use do not facilitate the progress for their effective control in foods. In the field of instrumental methods, the chromatographic ones coupled to different MS detectors (e.g., LC-MS/MS, LC-QTOF-MS, GC-MS) is still the most suitable means to detect contaminants in foods. There is a trend in employing both targeted and non-targeted high-resolution-MS methods (HRMS), which detect a wider array of compounds (multiclass)[55][56]. TOF accurate mass techniques can be applied for the non-targeted identification of pesticides, their metabolites, or degradation products and other unknown compounds present in the samples [57]. Cumbersome sample preparation is still a challenge that has to be resolved somehow in the future. Another challenge, is to reduce the solvent consumption during the course of analysis. More attention should be given to screening methods in order to reduce the population of samples to be analysed by separation methods or to promote the applicability of non-invasive methods of analysis. Smartphones can reform the present food testing status [58] by enabling farmers or consumers to examine the foods by themselves. Nonetheless, validation and benchmarking issues have to be considered carefully to verify method reliability, limit false-negative results, and that they are fit for the purpose.
2.3.3. Capacity Building in Assessing Biological Hazards
In line with the global One Health approach and in order to control/prevent human exposure to biological hazards through food, the European Commission has set up a comprehensive legal framework based on the scientific advice from EFSA to improve food safety in Europe. The efforts are focused on enhancing knowledge of pathogen origins and trends by monitoring zoonotic agents across the food and animal feed chain. Programs are developed to control Salmonella and other foodborne zoonotic disease and to reduce the risk to public health. In addition to human health risks, microbial contamination, which is assigned as a new hazard category that comes from ‘non-pathogenic microorganisms’, such as E. coli or Enterobacteriaceae, can result in food spoilage [59][60][61][62][63]. Emphasis was also given to the establishment of microbiological criteria that can be applied both at the different stages of food production and to food items already on the market, and on harmonisation of control measures against transmissible spongiform encephalopathies (TSE, BSE, scrapie) to prevent contagion of other animals or consumer exposure [63].
Conventional culture-based methods are the golden standards in food microbiology as they are considered to be simple, inexpensive and sensitive, though laborious and with important metrological constraint (false-negative results). METROFOOD-RI pays attention both to these reference methods as well as to alternative ones [64] for pre-processing and rapid/direct, target-specific culture-independent detection of foodborne pathogens in food samples, even at low level. Alternative methods may involve nucleic-acid-based methods (simple polymerase chain reaction (PCR), multiplex PCR, real-time PCR, nucleic acid sequence-based amplification (NASBA), loop-mediated isothermal amplification (LAMP), and oligonucleotide DNA microarray), immunological methods (enzyme-linked immunosorbent assay (ELISA) and lateral flow immunoassay), and biosensor-based methods (optical, electrochemical, and mass-based biosensors) [65][66]. Molecular typing by applying PCR techniques and sequencing has been developed rapidly worldwide [67]. According to the EFSA opinion of 2019 for Whole Genome Sequencing (WGS) [68], “WGS offers the highest level of bacterial strain discrimination for foodborne outbreak investigation and source-attribution as well as potential for more precise hazard identification, thereby facilitating more targeted risk assessment and risk management. WGS improves linking of sporadic cases associated with different food products and geographical regions to a point source outbreak and can facilitate epidemiological investigations, allowing also the use of previously sequenced genomes”.
Due to the nature of the analyte (i.e., living microorganism) and the different principles of the various methods used in food microbiology, harmonisation of methods through standardisation is of high importance to ensure comparability of results and to allow data exchange. Comprehensive testing and validation of alternative methods by collaborative studies will support the future development of a new generation of reliable foodborne pathogen detection technologies [68][69].The multi-omics approach integrating proteomics, metabolomics, metagenomics, and transcriptomics has also been proposed for the development of analytical methods to detect food pathogens rapidly in food and also to define potential biomarkers. In this direction, validation of biomarkers should be considered as the next most important stage within METROFOOD-RI capacity building for addressing microbial safety [70].
2.3.4. Perspective
Establishing competence of METROFOOD-RI in this area seems to be a priority given that each contaminant has to be determined in a variety of food products. METROFOOD-RI experts need to collaborate in proficiency testing in order to validate matrix-specific methods. Benchmarking toward instrumental methods and the emergence of smartphone-based methods for the detection of food contaminants should be one of the priorities among experts in the RI40 technology. Automation and IT tools from the e-component of the RI can be combined with the facilities of the Physical-RI to reduce time and cost and favour simplicity. In addition to the existing capability for standardisation of data generation and data analysis, upgrading e-platforms for data sharing will be also valuable in the direction of global control of food hazards (e.g., foodborne pathogens [71]).
2.4. Food Quality
2.4.1. What and How We ‘Measure’ It
The concept of ‘food quality’ encompasses numerous intrinsic and extrinsic characteristics of a food (raw materials, semi-processed/processed products) and all activities/services that take place from farm to fork. All interested parties (scientists, industry, authorities, consumers) influence decisions on the number of key quality attributes for each food product/commodity that should be considered at a given time period. Then, these attributes are either covered by legislation or become part of the internal specification armoury of each production unit. Quality control and quality management systems play a crucial role in the establishment of a product in the market. A product of ‘excellent’ quality should fulfil all specifications and consumer expectations including ‘extra’ benefits in comparison to any other product of its kind that can be found in the market. Any judgment about the degree of compliance of a product with a standard should be in a ‘non-arbitrary’ and impartial way so that it is of utmost importance to ’quantify’ quality attributes in an objective manner.
2.4.2. Perspective
The analytical labs of METROFOOD-RI cover well the feasibility of relevant physicochemical, microbiological/biological and organoleptic analysis of foods. In addition, following metrological concepts including, among others, high-quality reference standards, validated methods, robust sampling practices, proven calibration approaches, natural matrix-reference materials, speciation chemistry, assessment of measurement uncertainty and proficiency testing, the consortium may provide reliable data that can solve problems facilitating national/regional development, trade, and public health decisions, and contribute to nutrition education.
Τhis part of the scientific plan encompasses the emerging trend of using modern high-throughput non-destructive instruments, often called ’food scanners’ such as those based on spectroscopic methods. These devices are expected to facilitate numerous food sample testing procedures, thanks to minute sample amount and minimal or no sample pre-treatment, the availability of advanced chemometrics data handling tools, wireless data communication and ‘Big Data’ compatibility. Smartphone applications will assist near-future expectations such as the ability of food inspectors, farmers, retailers and consumers to test the quality characteristics of foods on their own [72]. On the other hand, processing facilities and kitchen-labs of the infrastructure supported by sensory panels have the expertise to contribute to the quality control and acceptability of reformulated or new products. Sensory analysis approaches that rely on well-trained panellists, even when standards are available, may introduce uncertainty issues because individuals exhibit different sensitivities, preferences, and product knowledge. The development of instrumental techniques that could recognise objectively and quickly specific sensory characteristics in the same way as an expert tasting panel perceive them is, thus, in high demand. Except for Gas Chromatography Olfactometry (GCO), biomimetic sensors such as electronic tongue (e-tongue), electronic nose, (e-nose), electronic eye, (e-eye) and computer vision systems (CVSs) are some of the available tools [29] that METROFOOD examines from the metrological point of view.
2.5. Nutritive Quality and Functional Properties
2.5.1. Nutrients and Beyond
In the light of a worldwide change in human consumption habits, accompanied by an increase in diet-related diseases primarily in industrialised nations—while hunger and malnutrition are rampant in most developing countries—the aspects of quality and nutritive value of food have become of central importance in nutrition research. Food wholesomeness is determined by the quantity and quality of nutrients contained therein and their harmlessness for human health [73]. Fresh food is widely purchased by consumers who can afford it as it is considered a healthier choice. Particularly for processed foods, nutrition-conscious consumers are paying increasing attention to the ingredients and are demanding high-value and healthy foods, even though the interpretation and definition of the term ‘healthy food’ is not consistently agreed upon experts [74]. Functional foods are a promising segment of the agri-business, even if this term still lacks a universally accepted definition for these products. Generally, they are defined as foods offering additional benefits that may reduce the risk of disease or promote optimal health when they are part of an everyday diet. In the face of increased health care costs related also to a higher occurrence of pathologies correlated with poor eating habits, clinical and epidemiological studies show that a healthy and balanced diet, rich in fruits, vegetables, whole grains, fish, and dairy products, and low in saturated fat and sodium, brings numerous benefits and can reduce the risk of diseases such as cardiovascular disease, hypertension and some types of cancer [74]. The introduction and exploitation of functional foods represents a great opportunity for the re-evaluation of traditional food products as well as the development of new products rich in bioactive substances. In order to ensure that any claim made on a food label is clear and substantiated by scientific evidence, European authorities issued the EC Regulation 1924/2006 concerning the use of nutrition and health claims. EFSA must verify the scientific substantiation of the petitions that have to fulfil all requirements according to specific guidelines [75].
2.5.2. State-of-the-Art Analytical Tools
As the determinants of general nutritive quality, proximates such as lipids, carbohydrates, proteins, and minerals have to be considered. For these, the golden standards are general reference methods collected under the ISO system. For trace nutrients such as elements and vitamins, the methodology is much less standardised and under on-going development. As trace quantitation is more susceptible to analytical errors and statistical imprecision, the methods require increased sensitivity and specificity, which is effectively achieved by mass spectrometric methods. However, although mass spectroscopy is highly specific and sensitive, signal intensity is often impeded by matrix effects. Moreover, extensive sample preparation often includes inferior recoveries, which require suitable compensation by internal standards. For this purpose, stable isotopologues are the compounds of choice and the respective methods are termed stable isotope dilution assays (SIDA). The most critical bottleneck for using SIDAs is the availability of the stable isotope labelled standards, and, therefore, new SIDAs are mainly elaborated by scientists that are experienced in synthesising these compounds [78][79][80][81]. For the quantitation of health-promoting compounds and bioactivities, the method portfolio is even more diverse and less standardised. Different protocols such as the 1,1-diphenyl- 2-picrylhydrazyl radical (DPPH radical) assay, oxygen radical absorbance capacity (ORAC) or the ferric reducing antioxidant power (FRAP) assay are in use and their results are not comparable and often conflicting [82]. Other bioactivity assays require the use of cell cultures, and therefore the results are even more so only indicative. Examples for these types of bioactivities are antimicrobial, anti-inflammatory, and antihypertensive properties. Here, the demand for standardisation is even higher to make the results comparable, reproducible, and includable in databases.
2.5.3. Perspective
According to expert perceptions, METROFOOD-RI should address in its scientific plan the following topics as summarised in Table 3.
Table 3. High-priority topics in the scientific plan for nutritive quality and functional properties.
| Topic | |
|---|---|
| 1 | Exploration of the health benefits of nutrients with enhanced value and structural function, such as phytonutrients. |
| 2 | Development of new technologies need to be developed for fractionation, isolation, extraction, reformulation (e.g., low salt content), concentration and delivery of health-promoting ingredients. |
| 3 | Raising the volume of food, feed, and fibre by reducing waste from food processing and by-product recovery. Development of novel processes for production of food products with high-value components (superfoods) and development of new processing technologies to protect and concentrate nutrients such as phytonutrients, vitamins, and flavour/aroma phenols. |
| 4 | Development and implementation of methods to improve processing and end-product quality and rapid measurement techniques for functionality and nutrient prediction. |
| 5 | Development of healthy, flavourful, and value-added food products to both maximise health effects and combat nutrition-related diseases. |
| 6 | Research on new administration techniques for nutrients (e.g., probiotics, nano-emulsions) and development of new processing technologies for the identification, characterisation, stabilisation, and delivery of nutrients |
| 7 | Development of knowledge and insight into the interaction between bio-metabolism and nutrients/food interactions. |
| 8 | Fostering quality improvements through research on foods and feed with increased added value, improving the quality of harvested and processed products, the quality of products in controlled atmospheres and reducing quality losses during storage. Establishing post-harvest practices toward optimising food quality through enhanced monitoring. |
For all these goals, accurate analytical methods for assessing the nutritive quality and health-promoting properties are essential.
The current methodological state of the art and the method portfolio available at METROFOOD-RI ensures the availability of laboratories quantifying carbohydrates, triacylglycerols/fatty acids and proteins/amino acids, as well as vitamins and minerals. Therefore, there is broad expertise within the consortium to assess the nutritive quality of foods and, by combining these labs with the facilities for RM production, to develop and produce new RMs fit for these analytical purposes. In the food production sector, METROFOOD-RI is highly specialised in food processing (e.g., development of pilot plants or innovative and mild food processing technologies), primary production (e.g., plants, livestock, seeds, feedstuffs), and food packaging technology (e.g., smart and active packaging solutions). With regard to nutritive quality, METROFOOD-RI already includes many analytical laboratories commonly applying general reference methods for proximates.
4. Conclusions
The overview of the latest scientific advancements in the wide key thematic areas where the METROFOOD-RI is active helps to highlight gaps and research trends in metrological issues related to food and nutrition that are of particular interest for upgrading the future services of the RI in the health and food domain. Summarising the research outcomes and views on each topic, it became possible to identify sub-targets of action. Horizontal activities aiming at increasing analytical testing competences, harmonising research methodologies, sharing of experience, best practices and networking, and fostering cross-border knowledge transfer through access to data/databases and analytical methods seem to be fundamental. Future goals of the METROFOOD-RI scientific plan include development of reliable and traceable diagnostic systems (methods and devices) and Reference Materials for quality, safety and traceability of raw materials and products; development of authenticity and traceability markers using targeted and non-targeted approaches; study of multiple exposure to different chemicals; emerging pesticides and mycotoxins; feed safety; microplastics; examination of effects of innovative technologies (e.g., nanotechnologies) to food quality and safety, and examination of the nutritive quality and wholesomeness of food with emphasis on nutrients and other functional constituents. METROFOOD-RI distributed facilities can face the current challenges of the agrifood sector and play a leading role in bringing together fragmented capabilities to form an integrated unit of excellence in the thematic areas articulated throughout the text.
This entry is adapted from the peer-reviewed paper 10.3390/foods11040599
References
- ESFRI Strategy Report on Research Infrastructures. Part 1. Contents. Available online: http://roadmap2018.esfri.eu/ (accessed on 2 December 2021).
- ESFRI Strategy Report on Research Infrastructures. Part 3. Projects and Landmarks. Available online: http://roadmap2018.esfri.eu/projects-and-landmarks (accessed on 2 December 2021).
- EURAMET. Strategic Research Agenda for Metrology in Europe; EURAMET e.V.: Braunschweig, Germany, 2016.
- Metrofood Infrastructure for Promoting Metrology in Food and Nutrition. Home Page. Available online: www.metrofood.eu (accessed on 2 December 2021).
- Rychlik, M.; Zappa, G.; Añorga, L.; Belc, N.; Castanheira, I.; Donard, O.F.X.; Kourimská, L.; Ogrinc, N.; Ocké, M.C.; Presser, K.; et al. Ensuring food integrity by metrology and FAIR data principles. Front. Chem. 2018, 6, 49.
- ISO Guide 30:2015; Reference Materials—Selected Terms and Definitions. International Organization for Standardization: Geneva, Switzerland, 2015.
- ISO 17034:2016; General Requirements for the Competence of Reference Material Producers. International Organization for Standardization: Geneva, Switzerland, 2016.
- Zappa, G.; Anorga, L.; Belc, N.; Castanheira, I.; Donard, O.F.X.; Kourimska, L.; Kukovecz, A.; Iatco, I.; Najdenkoska, A.; Nieminen, J.; et al. METROFOOD-RI: A new reality to develop Reference Materials for the agrifood sector. J. Phys. Conf. Ser. 2018, 1065, 232006.
- Zoani, C.; Anorga, L.; Belc, N.; Castanheira, I.; Donard, O.F.X.; Kourimska, L.; Kukovecz, A.; Iatco, I.; Najdenkoska, A.; Ogrinc, N.; et al. Feasibility studies for new food matrix-Reference Materials. J. Phys. Conf. Ser. 2018, 1065, 232005.
- European Commission JRC Science Areas. Available online: https://ec.europa.eu/jrc/en/science-areas (accessed on 2 December 2021).
- Emteborg, H.; Florian, D.; Choquette, S.; Ellison, S.L.R.; Fernandes-Whaley, M.; Mackay, L.; McCarron, P.; Panne, U.; Sander, S.G.; Kim, S.K.; et al. Cooperation in publicly funded reference material production. Accredit. Qual. Assur. 2018, 23, 371–377.
- Wise, S.A.; Phillips, M.M. Evolution of reference materials for the determination of organic nutrients in food and dietary supplements—A critical review. Anal. Bioanal. Chem. 2019, 411, 97–127.
- Recknagel, S.; Koch, M.; Koppen, R.; Buttler, S.; Penk, S.; Mauch, T.; Sommerfeld, T.; Witt, A. Development of certified reference materials for the determination of cadmium and acrylamide in cocoa. Anal. Bioanal. Chem. 2020, 412, 4659–4668.
- de Carvalho Couto, C.; dos Santos, D.G.; Oliveira, E.M.M.; Freitas-Silva, O. Global situation of reference materials to assure coffee, cocoa, and tea quality and safety. TrAC-Trends Anal. Chem. 2021, 143, 116381.
- Mari, L.; Narduzzi, C.; Nordin, G.; Trapmann, S. Foundations of uncertainty in evaluation of nominal properties. Meas. J. Int. Meas. Confed. 2020, 152, 107397.
- ISO Guide 35:2017; Reference Materials—Guidance for Characterization and Assessment of Homogeneity and Stability. International Organization for Standardization: Geneva, Switzerland, 2017.
- Greenberg, R.R.; Bode, P.; De Nadai Fernandes, E.A. Neutron activation analysis: A primary method of measurement. Spectrochim. Acta-Part B At. Spectrosc. 2011, 66, 193–241.
- Bhat, S.; Emslie, K.R. Digital polymerase chain reaction for characterisation of DNA reference materials. Biomol. Detect. Quantif. 2016, 10, 47–49.
- Dunn, P.J.H.; Malinovsky, D.; Achtar, E.; Clarkson, C.; Goenaga-Infante, H. Systematic comparison of post-column isotope dilution using LC-CO-IRMS with qNMR for amino acid purity determination. Anal. Bioanal. Chem. 2019, 411, 7207–7220.
- Food Integrity Project. FoodIntegrity Handbook-A Guide to Food Authenticity Issues and Analytical Solutions; Morin, J.-F., Lees, M., Eds.; Eurofins Analytics France: Nantes, France, 2018; Volume 53, ISBN 978-2-9566303-1-9.
- AOAC International Food Authenticity Methods Program. Available online: https://www.aoac.org/scientific-solutions/food-authenticity-fraud/ (accessed on 23 September 2020).
- European Commission Knowledge Centre for Food Fraud and Quality. Available online: https://ec.europa.eu/knowledge4policy/food-fraud-quality_en (accessed on 23 September 2020).
- Ulberth, F. Tools to combat food fraud—A gap analysis. Food Chem. 2020, 330, 127044.
- Matthäus, B.; Willenberg, I.; Engert, S.; Steinberg, P. The German National Reference Centre for Authentic Food (NRZ-Authent). OCL-Oilseeds Fats Crop. Lipids 2019, 26, 11.
- Borràs, E.; Ferré, J.; Boqué, R.; Mestres, M.; Aceña, L.; Busto, O. Data fusion methodologies for food and beverage authentication and quality assessment—A review. Anal. Chim. Acta 2015, 891, 1–14.
- Granato, D.; Putnik, P.; Kovačević, D.B.; Santos, J.S.; Calado, V.; Rocha, R.S.; Da Cruz, A.G.; Jarvis, B.; Rodionova, O.Y.; Pomerantsev, A. Trends in chemometrics: Food authentication, microbiology, and effects of processing. Compr. Rev. Food Sci. Food Saf. 2018, 17, 663–677.
- Callao, M.P.; Ruisánchez, I. An overview of multivariate qualitative methods for food fraud detection. Food Control 2018, 86, 283–293.
- Galvez, J.F.; Mejuto, J.C.; Simal-Gandara, J. Future challenges on the use of blockchain for food traceability analysis. Trends Anal. Chem. 2018, 107, 222–232.
- Di Rosa, A.R.; Leone, F.; Cheli, F.; Chiofalo, V. Fusion of electronic nose, electronic tongue and computer vision for animal source food authentication and quality assessment–A review. J. Food Eng. 2017, 210, 62–75.
- Riedl, J.; Esslinger, S.; Fauhl-Hassek, C. Review of validation and reporting of non-targeted fingerprinting approaches for food authentication. Anal. Chim. Acta 2015, 885, 17–32.
- Lo, Y.T.; Shaw, P.C. DNA-based techniques for authentication of processed food and food supplements. Food Chem. 2018, 240, 767–774.
- Hong, E.; Lee, S.Y.; Jeong, J.Y.; Park, J.M.; Kim, B.H.; Kwon, K.; Chun, H.S. Modern analytical methods for the detection of food fraud and adulteration by food category. J. Sci. Food Agric. 2017, 97, 3877–3896.
- Danezis, G.P.; Tsagkaris, A.S.; Camin, F.; Brusic, V.; Georgiou, C.A. Food authentication: Techniques, trends & emerging approaches. TrAC-Trends Anal. Chem. 2016, 85, 123–132.
- Bianchi, F.; Giannetto, M.; Careri, M. Analytical systems and metrological traceability of measurement data in food control assessment. Trends Anal. Chem. 2018, 107, 142–150.
- Tsimidou, M.Z.; Ordoudi, S.A.; Nenadis, N.; Mourtzinos, I. Food fraud. In Encycl. Food Health, 2nd ed.; Caballero, B., Finglas, P., Toldrá, F., Eds.; Academic Press: Oxford, UK, 2016; Volume 3, pp. 35–42.
- Creydt, M.; Fischer, M. Food authentication in real life: How to link nontargeted approaches with routine analytics? Electrophoresis 2020, 41, 1665–1679.
- Medina, S.; Perestrelo, R.; Silva, P.; Pereira, J.A.M.; Câmara, J.S. Current trends and recent advances on food authenticity technologies and chemometric approaches. Trends Food Sci. Technol. 2019, 85, 163–176.
- Ellis, D.I.; Muhamadali, H.; Haughey, S.A.; Elliott, C.T.; Goodacre, R. Point-and-shoot: Rapid quantitative detection methods for on-site food fraud analysis—Moving out of the laboratory and into the food supply chain. Anal. Methods 2015, 7, 9401–9414.
- Sobolev, A.P.; Thomas, F.; Donarski, J.; Ingallina, C.; Circi, S.; Cesare Marincola, F.; Capitani, D.; Mannina, L. Use of NMR applications to tackle future food fraud issues. Trends Food Sci. Technol. 2019, 91, 347–353.
- Hassoun, A.; Måge, I.; Schmidt, W.F.; Temiz, H.T.; Li, L.; Kim, H.-Y.; Nilsen, H.; Biancolillo, A.; Aït-Kaddour, A.; Sikorski, M.; et al. Fraud in Animal Origin Food Products: Advances in Emerging Spectroscopic Detection Methods over the Past Five Years. Foods 2020, 9, 1069.
- Oliveira, M.M.; Cruz-Tirado, J.P.; Barbin, D.F. Nontargeted analytical methods as a powerful tool for the authentication of spices and herbs: A review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 670–689.
- Böhme, K.; Calo-Mata, P.; Barros-Velázquez, J.; Ortea, I. Review of recent DNA-based methods for main food-authentication topics. J. Agric. Food Chem. 2019, 67, 3854–3864.
- Ruiz Orduna, A.; Husby, E.; Yang, C.T.; Ghosh, D.; Beaudry, F. Detection of meat species adulteration using high-resolution mass spectrometry and a proteogenomics strategy. Food Addit. Contam.-Part A Chem. Anal. Control. Expo. Risk Assess. 2017, 34, 1110–1120.
- Khalil, I.; Hashem, A.; Nath, A.R.; Muhd, N.; Yehye, W.A.; Jeffrey, W. DNA/Nano based advanced genetic detection tools for authentication of species: Strategies, prospects and limitations. Mol. Cell. Probes 2021, 59, 101758.
- European Commission. Report from the European Commission to the European Parliament and the Council on the Implementation of Regulation (EC) No 1185/2009 of the European Parliament and of the Council of 25 November 2009 Concerning Statistics on Pesticides; 2017. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:52017DC0109&from=en (accessed on 18 November 2021).
- European Court of Auditors. Special Report Sustainable Use of Plant Protection Products: Limited Progress in Measuring and Reducing Risks. Available online: https://www.eca.europa.eu/en/Pages/DocItem.aspx?did=53001 (accessed on 18 November 2021).
- European Parliament and Council. Regulation (EC) No 1107/2009 of the European Parliament and of the Council of 21 October 2009 Concerning the Placing of Plant Protection Products on the Market and Repealing Council Directives 79/117/EEC and 91/414/EEC. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32009R1107 (accessed on 18 November 2021).
- WHO Food Safety-Key Facts. Available online: https://www.who.int/news-room/fact-sheets/detail/food-safety (accessed on 2 December 2021).
- European Commission Food Safety. Contaminants. Available online: https://ec.europa.eu/food/safety/chemical-safety/contaminants_en (accessed on 2 December 2021).
- European Commission. The Rapid Alert System for Food and Feed 2017 Annual Report; Publications Office of the European Union: Luxembourg, 2017.
- European Commission. The Rapid Alert System for Food and Feed 2018 Annual Report; Publications Office of the European Union: Luxembourg, 2018.
- Vandermeersch, G.; Lourenço, H.M.; Alvarez-Muñoz, D.; Cunha, S.; Diogène, J.; Cano-Sancho, G.; Sloth, J.J.; Kwadijk, C.; Barcelo, D.; Allegaert, W.; et al. Environmental contaminants of emerging concern in seafood-European database on contaminant levels. Environ. Res. 2015, 143, 29–45.
- He, D.; Luo, Y.; Lu, S.; Liu, M.; Song, Y.; Lei, L. Microplastics in soils: Analytical methods, pollution characteristics and ecological risks. TrAC-Trends Anal. Chem. 2018, 109, 163–172.
- Usman, S.; Razis, A.F.A.; Shaari, K.; Amal, M.N.A.; Saad, M.Z.; Isa, N.M.; Nazarudin, M.F.; Zulkifli, S.Z.; Sutra, J.; Ibrahim, M.A. Microplastics pollution as an invisible potential threat to food safety and security, policy challenges and the way forward. Int. J. Environ. Res. Public Health 2020, 17, 9591.
- Steiner, D.; Malachová, A.; Sulyok, M.; Krska, R. Challenges and future directions in LC-MS-based multiclass method development for the quantification of food contaminants. Anal. Bioanal. Chem. 2021, 413, 25–34.
- Rausch, A.-K.; Brockmeyer, R.; Schwerdtle, T. Development, validation, and application of a multi-method for the determination of mycotoxins, plant growth regulators, tropane alkaloids, and pesticides in cereals by two-dimensional liquid chromatography tandem mass spectrometry. Anal. Bioanal. Chem. 2021, 413, 3041–3054.
- Ferrer, I.; Thurman, E. Application of LC–MS/MS and LC–TOF-MS for the Identification of Pesticide Residues and Their Metabolites in Environmental Samples. In Mass Spectrometry for the Analysis of Pesticide Residues and Their Metabolites; Tsipi, D., Botitsi, H., Economou, A., Eds.; John Wiley & Sons, Inc.: Hpboken, NJ, USA, 2015; pp. 207–230. ISBN 978-1-118-50017-0.
- Rateni, G.; Dario, P.; Cavallo, F. Smartphone-based food diagnostic technologies: A review. Sensors 2017, 17, 1453.
- Tsagkaris, A.S.; Nelis, J.L.D.; Ross, G.M.S.; Jafari, S.; Guercetti, J.; Kopper, K.; Zhao, Y.; Rafferty, K.; Salvador, J.P.; Migliorelli, D.; et al. Critical assessment of recent trends related to screening and confirmatory analytical methods for selected food contaminants and allergens. TrAC-Trends Anal. Chem. 2019, 121, 115688.
- Bintsis, T. Foodborne pathogens. AIMS Microbiol. 2017, 3, 529–563.
- WHO. Estimates of the Global Burden of Foodborne Diseases: Foodborne Disease Burden Epidemiology Reference Group 2007–2015; WHO Press: Geneva, Switzerland, 2015.
- Bosch, A.; Gkogka, E.; Le Guyader, F.S.; Loisy-Hamon, F.; Lee, A.; van Lieshout, L.; Marthi, B.; Myrmel, M.; Sansom, A.; Schultz, A.C.; et al. Foodborne viruses: Detection, risk assessment, and control options in food processing. Int. J. Food Microbiol. 2018, 285, 110–128.
- European Comission Biological Safety-Food Safety. Available online: https://ec.europa.eu/food/safety/biosafety_en (accessed on 2 October 2021).
- U.S. Food & Drug Administration (FDA). New Era of Smarter Food Safety. FDA´s Blueprint for the Future; FDA: Silver Spring, MD, USA, 2020.
- ISO 16140:2003; Microbiology of Food and Animal Feeding Stuffs—Protocol for the Validation of Alternative Methods. International Organization for Standardization: Geneva, Switzerland, 2016.
- Wang, Y.; Salazar, J.K. Culture-Independent Rapid Detection Methods for Bacterial Pathogens and Toxins in Food Matrices. Compr. Rev. Food Sci. Food Saf. 2016, 15, 183–205.
- Baraketi, A.; Salmieri, S.; Lacroix, M. Foodborne pathogens detection: Persevering worldwide challenge. In Biosensing Technologies for the Detection of Pathogens-A Prospective Way for Rapid Analysis; IntechOpen Limited: London, UK, 2018.
- EFSA. Panel on Biological Hazards Whole genome sequencing and metagenomics for outbreak investigation, source attribution and risk assessment of food-borne microorganisms. EFSA J. 2019, 17, 5898.
- Lombard, B.; Leclercq, A. The Role of Standardization Bodies in the Harmonization of Analytical Methods in Food Microbiology. In Global Issues in Food Science and Technology; Elsevier Academic Press: Burlington, UK, 2009; pp. 177–197. ISBN 9780123741240.
- Lüth, S.; Kleta, S.; Al Dahouk, S. Whole genome sequencing as a typing tool for foodborne pathogens like Listeria monocytogenes–The way towards global harmonisation and data exchange. Trends Food Sci. Technol. 2018, 73, 67–75.
- European Commission. Standing Committee on Plants Animals Food and Feed. Vision Paper on the Development of Data Bases for Molecular Testing of Foodborne Pathogens in View of Outbreak Preparedness. Available online: https://ec.europa.eu/food/safety/biological-safety/crisis-preparedness-management_el (accessed on 2 October 2021).
- Perrotta, G.; Donini, M.; Demurtas, O.C.; Mengoni, A. Integration of multi-omics data for biomarker identification of food safety and quality. In Science within Food: Up-to-Date Advances on Research and Educational Ideas; Formatex Research Center: Badajoz, Spain, 2017; pp. 152–163.
- Martínez-Gómez, J.; Ibarra, D.; Villacis, S.; Cuji, P.; Cruz, P.R. Analysis of LPG, electric and induction cookers during cooking typical Ecuadorian dishes into the national efficient cooking program. Food Policy 2016, 59, 88–102.
- Gaspar, M.C.M.P.; Garcia, A.M.; Larrea-Killinger, C. How would you define healthy food? Social representations of Brazilian, French and Spanish dietitians and young laywomen. Appetite 2020, 153, 104728.
- de Boer, A. Fifteen years of regulating Nutrition and Health Claims in Europe: The past, the present and the future. Nutrients 2021, 13, 1725.
- Nolvachai, Y.; Kulsing, C.; Marriott, P.J. Multidimensional gas chromatography in food analysis. TrAC Trends Anal. Chem. 2017, 96, 124–137.
- Guan, Z.W.; Yu, E.Z.; Feng, Q. Soluble dietary fiber, one of the most important nutrients for the gut microbiota. Molecules 2021, 26, 6802.
- Bachmann, T.; Maurer, A.; Rychlik, M. Development of a LC-MS/MS method using stable isotope dilution for the quantification of individual B6 vitamers in fruits, vegetables, and cereals. Anal. Bioanal. Chem. 2020, 412, 7237–7252.
- Striegel, L.; Chebib, S.; Netzel, M.E.; Rychlik, M. Improved Stable Isotope Dilution Assay for Dietary Folates Using LC-MS/MS and Its Application to Strawberries. Front. Chem. 2018, 6, 11.
- Ložnjak Švarc, P.; Oveland, E.; Strandler, H.S.; Kariluoto, S.; Campos-Giménez, E.; Ivarsen, E.; Malaviole, I.; Motta, C.; Rychlik, M.; Striegel, L.; et al. Collaborative study: Quantification of total folate in food using an efficient single-enzyme extraction combined with LC-MS/MS. Food Chem. 2020, 333, 127447.
- Wang, M.; Asam, S.; Chen, J.; Rychlik, M. Development of stable isotope dilution assays for the analysis of natural forms of vitamin B12 in meat. J. Agric. Food Chem. 2021, 69, 10722–10730.
- Zheng, L.; Lin, L.; Su, G.; Zhao, Q.; Zhao, M. Pitfalls of using 1,1-diphenyl-2-picrylhydrazyl (DPPH) assay to assess the radical scavenging activity of peptides: Its susceptibility to interference and low reactivity towards peptides. Food Res. Int. 2015, 76, 359–365.
This entry is offline, you can click here to edit this entry!
