Head and neck cancers rank sixth among the most common cancers today, and the survival rate has remained virtually unchanged over the past 25 years, due to late diagnosis and ineffective treatments. They have two main risk factors, tobacco and alcohol, and human papillomavirus infection is a secondary risk factor. These cancers affect areas of the body that are fundamental for the five senses. Therefore, it is necessary to treat them effectively and non-invasively as early as possible, in order to do not compromise vital functions, which is not always possible with conventional treatments (chemotherapy or radiotherapy).
- head and neck cancer
- nanomedicine
- drug delivery
- nanocarrier
1. Introduction
2. Anatomophysiology of the Head and Neck
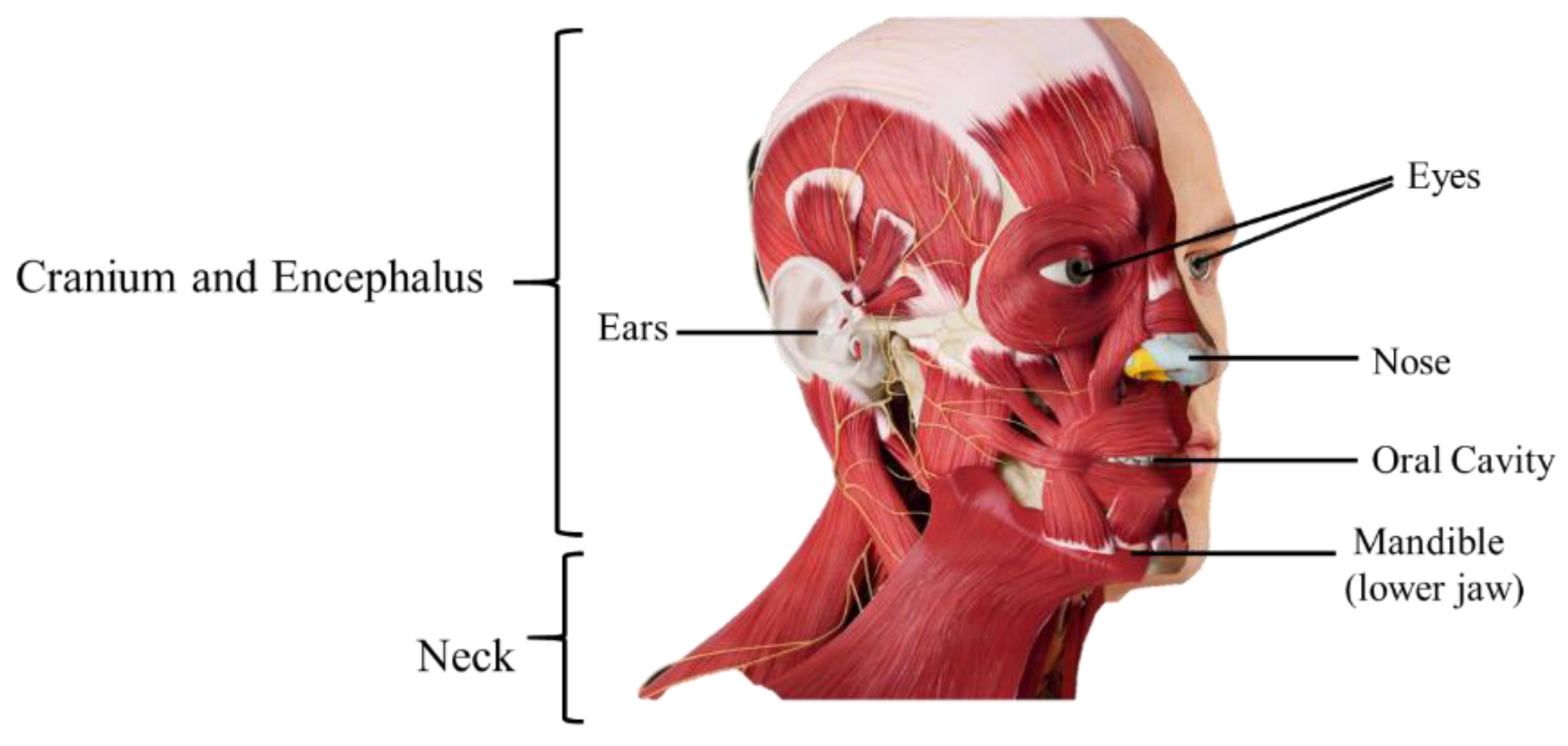
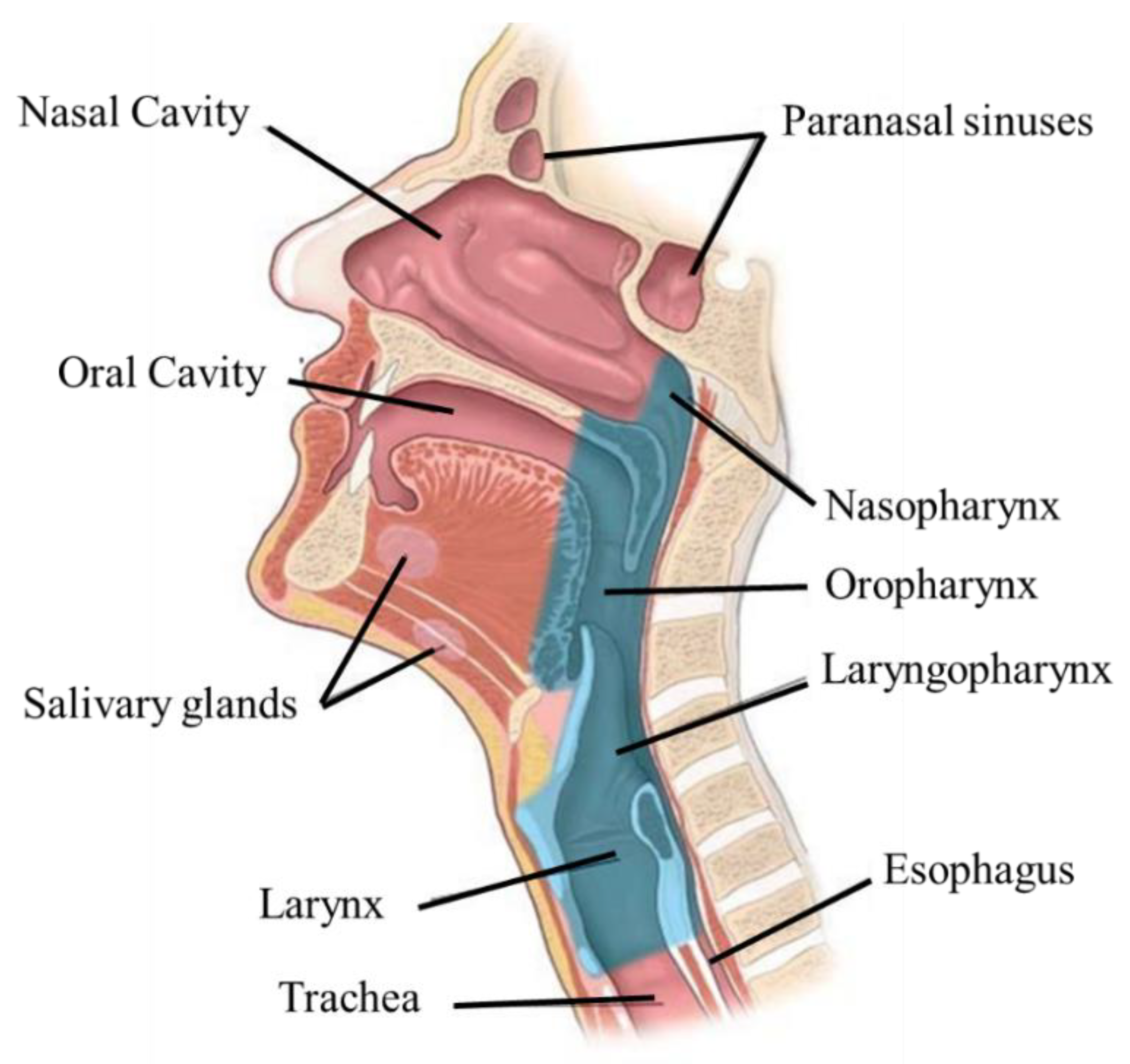
3. Head and Neck Cancer
3.1. Epidemiology, Etiology, and Risk Factors
3.2. Pathophysiology
3.3. Signs and Symptoms
3.4. Diagnosis and Treatment
| Stage | T | N | M |
|---|---|---|---|
| 0 | Defined shape | No invasion | No distant metastasis |
| I | Defined shape, less than 2 cm Does not invade the submucosa |
No invasion | No distant metastasis |
| II | Between 2 and 4 cm Initial invasion of the submucosa |
No invasion | No distant metastasis |
| III | Cancer cells rapidly divide Tumors with more than 4 cm |
Invasion | No distant metastasis |
| IV | Cancer cells enter the bloodstream | Invasion | Distant metastasis |
-
Radiation enhancement/synchronous chemotherapy, used in conjunction with radiation therapy—reduces the risk of lymph node metastasis;
-
Neo-adjuvant/induction chemotherapy—to reduce the tumor’s size before the main treatment;
-
Adjuvant—acts on the small lesions that cannot be removed by surgery, reduces the recurrence rate, and improves the survival rate;
-
Palliation—for distant metastases.
4. Nanomedicine as a Therapeutic Approach for HNC
-
The improvement of diagnosis and the development of novel strategies for early detection of tumors, circulating tumor cells, and metastases;
-
The improvement of treatment of solid tumors and chemo-resistant tumors;
-
The improved precision and efficacy of radiotherapy, immunotherapy, photodynamic, individualized, and hyperthermia therapies
-
Lower side effects through more targeted chemotherapy.
4.1. Passive Targeting
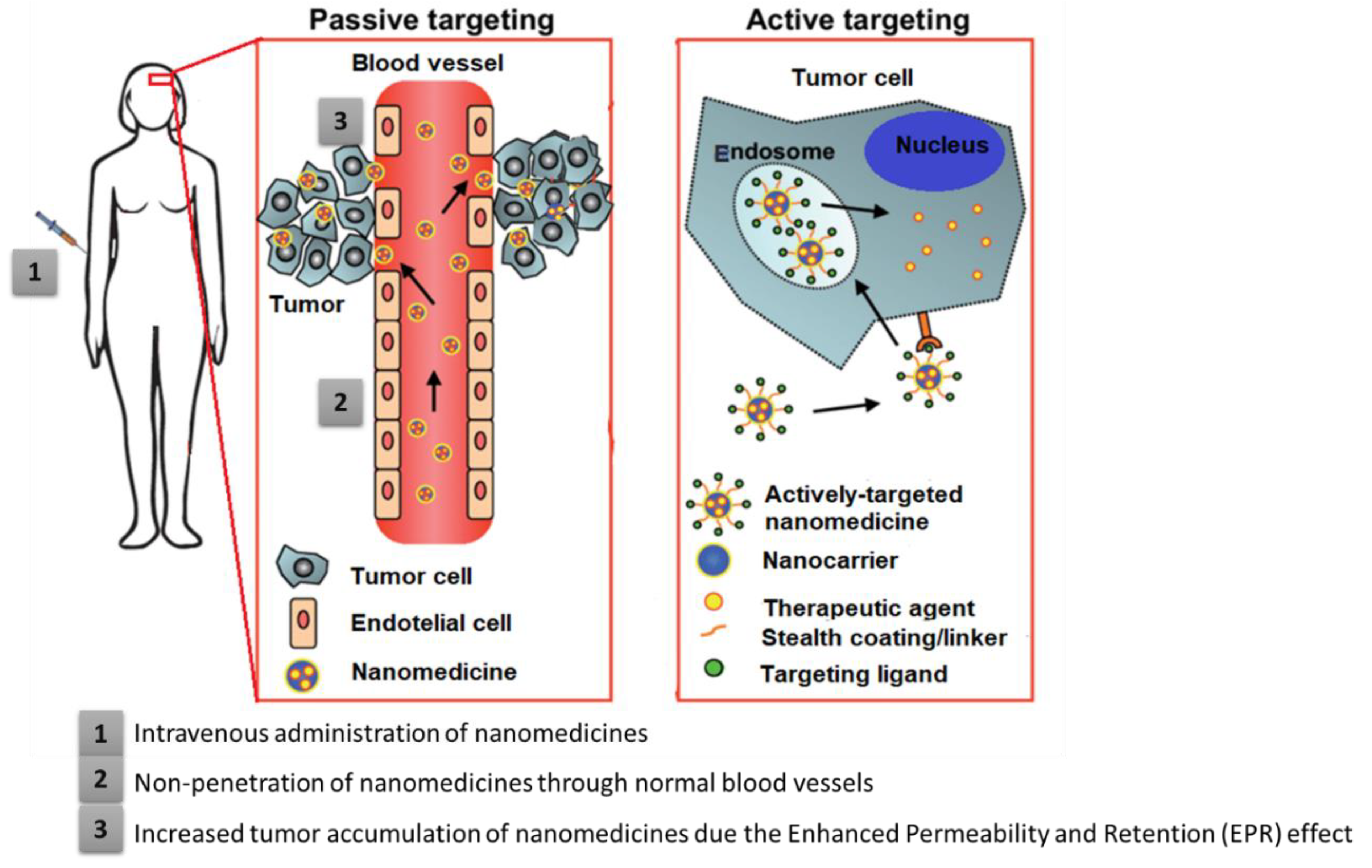
4.2. Active Targeting
This entry is adapted from the peer-reviewed paper 10.3390/ma15062086
References
- World Health Organization (WHO)—Cancer Control Programme. Available online: http://www.who.int/cancer/en/ (accessed on 20 December 2021).
- Nayak, M.G.; George, A.; Vidyasagar, M.S.; Mathew, S.; Nayak, S.; Nayak, B.S.; Shashidhara, Y.N.; Kamath, A. Quality of life among cancer patients. Indian J. Palliat. Care 2017, 23, 445.
- Weizman, B.; Golan, N.; Ronen, O. Effect of socioeconomic status on survival in patients with head and neck cancer. Head Neck 2021, 43, 3001–3009.
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249.
- Fidler, M.M.; Bray, F.; Soerjomataram, I. The global cancer burden and human development: A review. Scand. J. Public Health 2018, 46, 27–36.
- Rezende, T.M.; de Souza Freire, M.; Franco, O.L. Head and neck cancer: Proteomic advances and biomarker achievements. Cancer 2010, 116, 4914–4925.
- American Cancer Society. Cancer Facts & Figures. 2021. Available online: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2021.html (accessed on 11 January 2022).
- Birkeland, A.C.; Swiecicki, P.L.; Brenner, J.C.; Shuman, A.G. A review of drugs in development for the personalized treatment of head and neck squamous cell carcinoma. Expert Rev. Precis. Med. Drug Dev. 2016, 1, 379–385.
- de Lázaro, I.; Mooney, D.J. Obstacles and opportunities in a forward vision for cancer nanomedicine. Nat. Mater. 2021, 20, 1469–1479.
- Kurmi, B.D.; Patel, P.; Paliwal, R.; Paliwal, S.R. Molecular approaches for targeted drug delivery towards cancer: A concise review with respect to nanotechnology. J. Drug Deliv. Sci. Technol. 2020, 57, 101682.
- Zhao, Y.; Chen, H.; Chen, X.; Hollett, G.; Gu, Z.; Wu, J.; Liu, X. Targeted nanoparticles for head and neck cancers: Overview and perspectives. WIREs Nanomed. Nanobiotechnology 2017, 9, e1469.
- Gharat, S.A.; Momin, M.M.; Bhavsar, C. Oral squamous cell carcinoma: Current treatment strategies and nanotechnology-based approaches for prevention and therapy. Crit. Rev. Ther. Drug Carr. Syst. 2016, 33, 363–400.
- Bharadwaj, R.; Medhi, S. Oral squamous cell carcinoma and the cutting edge of nanotechnology. Multidiscip. Cancer Investig. 2020, 4, 36–45.
- El-Say, K.M.; El-Sawy, H.S. Polymeric nanoparticles: Promising platform for drug delivery. Int. J. Pharm. 2017, 528, 675–691.
- Dinda, S.C.; Pattnaik, G. Nanobiotechnology-based drug delivery in brain targeting. Curr. Pharm. Biotechnol. 2013, 14, 1264–1274.
- Chen, X.-J.; Zhang, X.-Q.; Liu, Q.; Zhang, J.; Zhou, G. Nanotechnology: A promising method for oral cancer detection and diagnosis. J. Nanobiotechnology 2018, 16, 52.
- Wojtynek, N.E.; Mohs, A.M. Image-guided tumor surgery: The emerging role of nanotechnology. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2020, 12, e1624.
- Mooore, K.L.; Dalley, A.F.; Agur, A.M.R. Moore Clinically Oriented Anatomy; Wolters Kluwer India Pvt Ltd.: Gurugram, India, 2017.
- Betts, J.G.; Y, K.A.; Wise, J.A.; Johnson, E.; Poe, B.; Kruse, D.H.; Korol, O.; Johnson, J.E.; Womble, M.; DeSaix, P. 7.2 The skull. In Anatomy and Physiology; OpenStax: Houston, TX, USA, 2013.
- Roesch, Z.K.; Tadi, P. Anatomy of head and neck. In StatPearls; StatPearls Publishing Copyright © 2020; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2020.
- Lester, S.; Yang, W.-Y. Principles and management of head and neck cancer. Surgery 2012, 30, 617–623.
- Crozier, E.; Sumer, B.D. Head and neck cancer. Med. Clin. N. Am. 2010, 94, 1031–1046.
- Bose, P.; Brockton, N.T.; Dort, J.C. Head and neck cancer: From anatomy to biology. Int. J. Cancer 2013, 133, 2013–2023.
- Johnson, N.W.; Amarasinghe, H.K. Epidemiology and aetiology of head and neck cancers. In Head and Neck Cancer; Bernier, J., Ed.; Springer: Geneva, Switzerland, 2011; p. 730.
- Kawashita, Y.; Soutome, S.; Umeda, M.; Saito, T. Oral management strategies for radiotherapy of head and neck cancer. Jpn. Dent. Sci. Rev. 2020, 56, 62–67.
- Wu, T.T.; Zhou, S.H. Nanoparticle-based targeted therapeutics in head-and-neck cancer. Int. J. Med. Sci. 2015, 12, 187–200.
- Gao, S.; Zheng, M.; Ren, X.; Tang, Y.; Liang, X. Local hyperthermia in head and neck cancer: Mechanism, application and advance. Oncotarget 2016, 7, 57367–57378.
- Argiris, A.; Karamouzis, M.V.; Raben, D.; Ferris, R.L. Head and neck cancer. Lancet 2008, 371, 1695–1709.
- Pezzuto, F.; Buonaguro, L.; Caponigro, F.; Ionna, F.; Starita, N.; Annunziata, C.; Buonaguro, F.M.; Tornesello, M.L. Update on head and neck cancer: Current knowledge on epidemiology, risk factors, molecular features and novel therapies. Oncology 2015, 89, 125–136.
- Aupérin, A. Epidemiology of head and neck cancers: An update. Curr. Opin. Oncol. 2020, 32, 178–186.
- Hashibe, M.; Brennan, P.; Chuang, S.C.; Boccia, S.; Castellsague, X.; Chen, C.; Curado, M.P.; Maso, L.D.; Daudt, A.W.; Fabianova, E.; et al. Interaction between tobacco and alcohol use and the risk of head and neck cancer: Pooled analysis in the international head and neck cancer epidemiology consortium. Cancer Epidemiol. Biomark. Prev. 2009, 18, 541–550.
- Chan, J.Y.K.; Zhen, G.; Agrawal, N. The role of tumor DNA as a diagnostic tool for head and neck squamous cell carcinoma. Semin. Cancer Biol. 2019, 55, 1–7.
- Zolkind, P.; Lee, J.J.; Jackson, R.S.; Pipkorn, P.; Massa, S.T. Untreated head and neck cancer: Natural history and associated factors. Head Neck 2021, 43, 89–97.
- Chua, M.L.K.; Wee, J.T.S.; Hui, E.P.; Chan, A.T.C. Nasopharyngeal carcinoma. Lancet 2016, 387, 1012–1024.
- Smatti, M.K.; Al-Sadeq, D.W.; Ali, N.H.; Pintus, G.; Abou-Saleh, H.; Nasrallah, G.K. Epstein–Barr virus epidemiology, serology, and genetic variability of LMP-1 oncogene among healthy population: An update. Front. Oncol. 2018, 8, 211.
- Al-Thawadi, H.; Gupta, I.; Jabeen, A.; Skenderi, F.; Aboulkassim, T.; Yasmeen, A.; Malki, M.I.; Batist, G.; Vranic, S.; Al Moustafa, A.-E. Co-presence of human papillomaviruses and Epstein–Barr virus is linked with advanced tumor stage: A tissue microarray study in head and neck cancer patients. Cancer Cell Int. 2020, 20, 361.
- Sroussi, H.Y.; Jessri, M.; Epstein, J. Oral assessment and management of the patient with head and neck cancer. Oral Maxillofac. Surg. Clin. 2018, 30, 445–458.
- Sahovaler, A.; Yeh, D.H.; Fung, K. General principles of head and neck cancer treatment. In Clinical Care and Rehabilitation in Head and Neck Cancer; Doyle, P.C., Ed.; Springer Nature: Cham, Switzerland, 2019; pp. 3–14.
- Vokes, E.E.; Weichselbaum, R.R.; Lippman, S.M.; Hong, W.K. Head and neck cancer. N. Engl. J. Med. 1993, 328, 184–194.
- Heroiu Cataloiu, A.-D.; Danciu, C.E.; Popescu, C.R. Multiple cancers of the head and neck. Maedica 2013, 8, 80–85.
- Chen, S.-W.; Li, S.-H.; Shi, D.-B.; Jiang, W.-M.; Song, M.; Yang, A.-K.; Li, Y.-D.; Bei, J.-X.; Chen, W.-K.; Zhang, Q. Expression of PD-1/PD-L1 in head and neck squamous cell carcinoma and its clinical significance. Int. J. Biol. Markers 2019, 34, 398–405.
- Lenouvel, D.; González-Moles, M.; Talbaoui, A.; Ramos-García, P.; González-Ruiz, L.; Ruiz-Ávila, I.; Gil-Montoya, J.A. An update of knowledge on PD-L1 in head and neck cancers: Physiologic, prognostic and therapeutic perspectives. Oral Dis. 2020, 26, 511–526.
- Stepnick, D.; Gilpin, D. Head and Neck Cancer—An Overview. Semin. Plast. Surg. 2009, 24, 107–116.
- Panikkanvalappil, S.R.; El-Sayed, M.A.; El-sayed, I.H. Advances in nanomedicine for head and neck cancer. In Head and Neck Cancer; Bernier, J., Ed.; Springer: Cham, Switzerland, 2016; pp. 827–843.
- Ordoñez, R.; Otero, A.; Jerez, I.; Medina, J.A.; Lupiañez-Pérez, Y.; Gomez-Millan, J. Role of radiotherapy in the treatment of metastatic head and neck cancer. OncoTargets Ther. 2019, 12, 677–683.
- Guidi, A.; Codecà, C.; Ferrari, D. Chemotherapy and immunotherapy for recurrent and metastatic head and neck cancer: A systematic review. Med. Oncol. 2018, 35, 37.
- Saloura, V.; Langerman, A.; Rudra, S.; Chin, R.; Cohen, E.E.W. Multidisciplinary care of the patient with head and neck cancer. Surg. Oncol. Clin. N. Am. 2013, 22, 179–215.
- Machiels, J.P.; René Leemans, C.; Golusinski, W.; Grau, C.; Licitra, L.; Gregoire, V. Squamous cell carcinoma of the oral cavity, larynx, oropharynx and hypopharynx: Clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 1462–1475.
- Ferrari, D.; Ghi, M.G.; Franzese, C.; Codecà, C.; Gau, M.; Fayette, J. The slippery role of induction chemotherapy in head and neck cancer: Myth and reality. Front. Oncol. 2020, 10, 7.
- Al Qaraghuli, M.M. Biotherapeutic antibodies for the treatment of head and neck cancer: Current approaches and future considerations of photothermal therapies. Front. Oncol. 2020, 10, 2710.
- Herskovic, A.; Martz, K.; Al-Sarraf, M.; Leichman, L.; Brindle, J.; Vaitkevicius, V.; Cooper, J.; Byhardt, R.; Davis, L.; Emami, B. Combined Chemotherapy and Radiotherapy Compared with Radiotherapy Alone in Patients with Cancer of the Esophagus. N. Engl. J. Med. 1992, 326, 1593–1598.
- Pillai, G. Nanotechnology Toward Treating Cancer. In Applications of Targeted Nano Drugs and Delivery Systems; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 221–256.
- Xu, Q.; Fang, M.; Zhu, J.; Dong, H.; Cao, J.; Yan, L.; Leonard, F.; Oppel, F.; Sudhoff, H.; Kaufmann, A.M. Insights into nanomedicine for immunotherapeutics in squamous cell carcinoma of the head and neck. Int. J. Biol. Sci. 2020, 16, 2506.
- Napolitano, M.; Schipilliti, F.M.; Trudu, L.; Bertolini, F. Immunotherapy in head and neck cancer: The great challenge of patient selection. Crit. Rev. Oncol./Hematol. 2019, 144, 102829.
- Wilczewska, A.Z.; Niemirowicz, K.; Markiewicz, K.H.; Car, H. Nanoparticles as drug delivery systems. Pharmacol. Rep. 2012, 64, 1020–1037.
- Chang, E.H.; Harford, J.B.; Eaton, M.A.W.; Boisseau, P.M.; Dube, A.; Hayeshi, R.; Swai, H.; Lee, D.S. Nanomedicine: Past, present and future—A global perspective. Biochem. Biophys. Res. Commun. 2015, 468, 511–517.
- Caruthers, S.D.; Wickline, S.A.; Lanza, G.M. Nanotechnological applications in medicine. Curr. Opin. Biotechnol. 2007, 18, 26–30.
- Soares, S.; Sousa, J.; Pais, A.; Vitorino, C. Nanomedicine: Principles, properties, and regulatory issues. Front. Chem. 2018, 6, 360.
- Muthu, M.S.; Mei, L.; Feng, S.-S. Nanotheranostics: Advanced nanomedicine for the integration of diagnosis and therapy. Nanomedicine 2014, 9, 1277–1280.
- Astruc, D. Introduction to Nanomedicine. Molecules 2016, 21, 4.
- Cormode, D.P.; Skajaa, T.; Fayad, Z.A.; Mulder, W.J.M. Nanotechnology in medical imaging: Probe design and applications. Arterioscler. Thromb. Vasc. Biol. 2010, 29, 992–1000.
- Rodrigues, A.R.O.; Almeida, B.G.; Rodrigues, J.M.; Queiroz, M.J.R.P.; Calhelha, R.C.; Ferreira, I.C.F.R.; Pires, A.; Pereira, A.M.; Araújo, J.P.; Coutinho, P.J.G.; et al. Magnetoliposomes as carriers for promising antitumor thienopyridin-7-arylamines: Photophysical and biological studies. R. Soc. Chem. Adv. 2017, 7, 15352–15361.
- Clarke, J.; Wu, H.C.; Jayasinghe, L.; Patel, A.; Reid, S.; Bayley, H. Continuous base identification for single-molecule nanopore DNA sequencing. Nat. Nanotechnol. 2009, 4, 265–270.
- Danie Kingsley, J.; Ranjan, S.; Dasgupta, N.; Saha, P. Nanotechnology for tissue engineering: Need, techniques and applications. J. Pharm. Res. 2013, 7, 200–204.
- Nanomedicine, E.T.P. Nanomedicine Strategic Research & Innovation Agenda 2016–2030: Creating Junctions for Healthcare; European Technology Platform for Nanomedicine: Paris, France, 2016; pp. 1–31.
- Caban, S.; Aytekin, E.; Sahin, A.; Capan, Y. Nanosystems for drug delivery. OA Drug Des. Deliv. 2014, 2, 1–7.
- Cho, K.; Wang, X.; Nie, S.; Chen, Z.; Shin, D.M. Therapeutic nanoparticles for drug delivery in cancer. Clin. Cancer Res. 2008, 14, 1310–1316.
- Nikalje, A.P. Nanotechnology and its applications in medicine. Med. Chem. 2015, 5, 81–89.
- Pradhan, P.; Giri, J.; Rieken, F.; Koch, C.; Mykhaylyk, O.; Döblinger, M.; Banerjee, R.; Bahadur, D.; Plank, C. Targeted temperature sensitive magnetic liposomes for thermo-chemotherapy. J. Control. Release 2010, 142, 108–121.
- Aghebati-Maleki, A.; Dolati, S.; Ahmadi, M.; Baghbanzhadeh, A.; Asadi, M.; Fotouhi, A.; Yousefi, M.; Aghebati-Maleki, L. Nanoparticles and cancer therapy: Perspectives for application of nanoparticles in the treatment of cancers. J. Cell. Physiol. 2020, 235, 1962–1972.
- Bertrand, N.; Wu, J.; Xu, X.; Kamaly, N.; Farokhzad, O.C. Cancer nanotechnology: The impact of passive and active targeting in the era of modern cancer biology. Adv. Drug Deliv. Rev. 2014, 66, 2–25.
- Peer, D.; Karp, J.M.; Hong, S.; Farokhzad, O.C.; Margalit, R.; Langer, R. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2007, 2, 751–760.
- Khan, D.R. The use of nanocarriers for drug delivery in cancer therapy. J. Cancer Sci. Ther. 2010, 2, 58–62.
- Inagaki, F.F.; Furusawa, A.; Choyke, P.L.; Kobayashi, H. Enhanced nanodrug delivery in tumors after near-infrared photoimmunotherapy. Nanophotonics 2019, 8, 1673–1688.
- Wu, J. The Enhanced Permeability and Retention (EPR) Effect: The Significance of the Concept and Methods to Enhance Its Application. J. Pers. Med. 2021, 11, 771.
- van der Meel, R. Targeted Inhibition of Tumor Growth and Angiogenesis. Ph.D. Thesis, Universiteit Utrecht, Utrecht, The Netherlands, 2013.
- Torchilin, V.P. Passive and active drug targeting: Drug delivery to tumors as an example. In Handbook of Experimental Pharmacology, 197th ed.; Springer: Berlin, Germany, 2010; Volume 197, pp. 3–53.
- Kudr, J.; Haddad, Y.; Richtera, L.; Heger, Z.; Cernak, M.; Adam, V.; Zitka, O. Magnetic nanoparticles: From design and synthesis to real world applications. Nanomaterials 2017, 7, 243.
- Pawar, P.V.; Domb, A.J.; Kumar, N. Systemic targeting systems. In Advances in Delivery Science and Technology; Springer: Boston, MA, USA, 2014; pp. 61–91.
