Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Biochemical Research Methods
Owing to the nanometer size range, Quantum Dots (QDs) have exhibited unique physical and chemical properties which are favourable for different applications. Especially, due to their quantum confinement effect, excellent optoelectronic characteristics is been observed. This considerable progress has not only uplifted the singular usage of QDs, but also encouraged to prepare various hybrid materials to achieve superior efficiency by eliminating certain shortcomings. Such issues can be overcome by compositing QDs with polymers.
- polymers
- Quantum Dots
- nanocomposites
- electrodes
- electrolytes
- synthesis
1. Electrochemical Process
Apart from hydrothermal and microwave irradiated QDs preparation, electrochemical process is another well known and widely studied technique. This process is one of the major categories of top down synthesis approach. Unlike other synthesis method, this process is been reported to be examined from several minute reaction duration to several days. However, few studies have mentioned its swift reaction period and lesser temperature requirement which can easily lead to scaling up of the material production [38]. For an instance, Li et al. prepared 4 nm, uniform, and monodispersed CQDs with strong and stable photoluminescence via employing graphite rods as both cathode and anode in NaOH/EtOH electrolyte where the author mentioned that production rate of CQDs was around 10 mg per hour for each processing setup [38]. Similar to CQDs, GQDs also can be synthesized in facile conditions. Li et al. utilized graphene sheet as an electrode with phosphate buffer solution to prepare water soluble GQDs of 3–5 nm size [39]. So, this process have appropriately exhibited electrochemical oxidation of the carbon precursors to synthesize CQDs as well as appropriate pathways for GQDs or doped GQDs [40,41]. By employing single-stage facile alternating voltage electrochemical technique, preparation of NiO quantum dots/graphene composite was completed where both Ni flake and Graphite rod were exfoliated via using alternating voltage of 5 V and NaOH (2 M) electrolyte was used [42]. With using Ni flake and graphite rod as electrodes, the as-prepared NiO quantum dots were uniformly dispersed on the surface of graphene and an enhanced electrochemical activity was achieved using the prepared composite electrode material. Preparation of WS2 and MoS2 via electrochemical technique is also been explored by many researchers [43]. Valappil et al. prepared WS2 QDs by synergistic effect of Lithium Perchlorate intercalation and propylene carbonate used as electrolyte [44]. Potential of 2 V was applied with the described electrochemical setup. The as-prepared WS2 QDs have average size of 3 nm with few-layers and exhibited PL emission (PLQY = 5%). Similarly, MoS2 QDs (size of 2.5 to 6 nm) were prepared via one step electrochemical method from its bulk material along with using diluted aqueous ionic liquid solutions of 1-butyl-3-methylimidazolium chloride and bis-trifluoromethylsulphonylimide [45]. As-prepared MoS2 QDs exhibited excitation dependent luminescence, which could be further enhanced via surface passivation.
So, this process have successfully shown advantages like enhanced production yield, flexible usage of electrolytes, superior stability without any additive binders during synthesis, efficient exfoliation via regulating the potential or current [43,46]. More importantly, size controllability is another crucial aspect which can be achieved with this process by altering temperature of electrolyte [40]. However, electrochemical process involves graphite or expensive carbon precursors for synthesis which raises overall production costs. Also, unnecessary attached residual additives on the surface of QDs have restricted efficiency of this method. Zhao et al. reported carbon quantum dots-polyaniline hybrid material, the PANI was grown on carbon fiber substrate resulting in a interconnected network structure as shown in Figure 1a [47]. Vandana et al. reported quantum dots dispersed on polymers synthesized through a facile hydrothermal method using glucose as source for quantum dots as shown in Figure 1b [48]. She et al. elaborated on the synthesis of graphene quantum dots and polypyrrole hybrids. The electrochemically reduced graphene was decorated on the polypyrrole sphere as shown in Figure 1c [49]. Li et al. reported core-shell@PANI using citric acid as precursor which follows pyrolysis and NaOH treatment, further the PANI was composited by in situ adsorption polymerization as described in the Figure 1d [50].
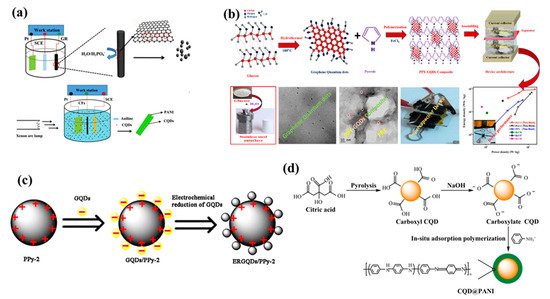
Figure 1. (a) Schematic illustrations of electrochemical fabrication of CQDs and CQDs-PANI/CF(LI), (b) A schematic diagram represents the synthesis mechanism of GQDs and PPY-GQDs composite fabricated as a supercapacitor device, (c) Schematic illustration showing the preparation of ERGQDs/PPy-2, (d) Schematic diagram of the preparation procedure of CQD@PANI nanoparticles. Reprinted with permission from: Ref. [47]. Copyright 2016 Elsevier; Ref. [48]. Copyright 2020 Elsevier; Ref. [51]. Copyright 2021 Elsevier; Ref. [52]. Copyright 2019 ACS.
2. Solvothermal/Hydrothermal Process
These synthesis procedures are very frequently employed not only for the synthesis for QDs, but also for different range of materials having various size ranges and configuration. Mostly, it involves single-step procedure in which organic precursors are treated in a closed autoclave to prepare the QDs under high pressure and temperature as per the requirement. Here selection of precursors plays a vital role because the source used may not only contain carbon but may also have doping elements which can impact the synthesized QDs structure. Citric acid, L-cysteine, melamine, hydrazine, Polyethylene glycol-400, (1, 3, 6)-trinitropyrene, hydrazine hydrate are often used as CQD precursors where as bromobenzoic acid, citric acid are the different precursors used in synthesis of GQDs. Other than these precursors CQDs are also been prepared via dehydration of glucose in sulfuric acid and nitric acid [40]. Additionally, the usage of further sulphur and nitrogen doping are observed to make better application outcome of the CQDs or GQDS. For doping purposes, nitrogen (N) and sulphur (S) sources included in the preparation are such as thiourea, urea, hexamethylenetetramine, ethylenediamine and diethyl diethanolamine which are used to synthesize N or S-doped QDs [40]. However, rather than focusing only on one step approach, researchers have adapted two-step synthesis approach to mainly obtain doped QDs with improved structure and applicability [53]. For an example, GQDs are prepared with citric acid taking as precursor with basic condition hydrothermal processes, and then the as-synthesized QDs are blended with hydrazine and heated for several hours to prepared N-doped QDs. Not only CQDs or GQDs, hydrothermal technique is also been used to prepare CeO2/Ce2O3 quantum dots anchored on reduced graphene oxide sheets of different weight fractions where graphene oxide was synthesized initially using natural graphite flakes via employing modified Hummer’s method [54]. Cerium nitrate hexahydrate was the precursor used in this preparation. Similarly, single-step solvothermal technique was also employed to synthesize paper-like layered CeO2 quantum dots doped Ni-Co hydroxide nanosheets which was used as electrode in asymmetric supercapacitor [55]. Utilizing citric acid, thiourea and ceria like precursors along with hydrothermal method, N and S co-doped graphene quantum dots were grown on CeO2 nanoparticles and this combination of ceria and graphene quantum dots were found to be providing more active surface area, hence enhanced electrochemical activity [56].
Hydrothermal method is also used to prepare CuO quantum dots via using copper (II) acetate monohydrate precursor and additionally single layer graphene was added on the CuO quantum dot surface for better structural stability (unique core-shell structure) and improved performance [57]. Likewise, CuS, SnO2, NiCo2O4 quantum dots were also produced using copper (II) dithiooxamide, stannous chloride dehydrate, nickel acetate tetra-hydrate and cobalt acetate tetra-hydrate precursors respectively [58,59,60]. In order to synthesize WS2 and MoS2 QDs, solvothermal technique is been broadly used [61]. Precursors like sodium molybdate and cysteine are been used to form MoS2 QDs where as sodium tungstate and L-glutathione were used to prepare WS2 QDs [62,63]. L-glutathione and cysteine is mainly used as the source for sulfide. However, studies have also mentioned the use of thiocarbamide, dibenzyldisulfide, thiourea as the sulfide source in synthesizing WS2 and MoS2 QDs [61]. The key benefits of this method are less hazardous, easier to conduct and less expensive. Moreover, controlling and altering the properties as well as composition of the prepared QDs is possible using this method, which are favorable for application purposes.
3. Microwave Synthesis
Compared to hydrothermal or solvothermal process, microwave irradiated preparation techniques consume less time and required lesser temperature for synthesizing QDs. Via adding water with glucose and ammonia, GQDs are been prepared under microwave irradiation which took only one minute reaction time [62,63]. Another group has prepared similar GQDs in just 5 min under 200 °C using acetylacetone along with water in quartz bowl kept under 800 W power microwave irradiation. Not only because of quicker reaction time and low temperature requirement, but also due to easy reaction route and overall economical factors this technique fits suitable for large scale production of GQDs as suggested by researchers [40,64]. The microwave synthesis of GQDs and its modification by tuning different parameters like power, time temperature etc. have been illustrated in the Figure 2 [65,66]. On top of that, in case of characteristics of CQDs or GQDs can be controlled and altered via adjusting irradiation power and reaction timing or by varying precursors. Although, using the microwave method for production of CQDs are not clearly mentioned in the literature to be completely apt.
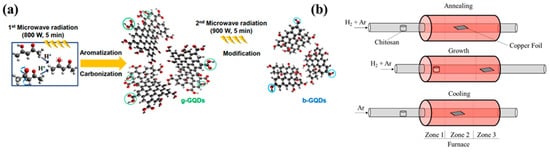
Figure 2. (a) Schematic illustration of microwave bottom-up route for GQDs and b-GQDs: green circles mean carboxyl and carbonyl groups and blue circles indicate hydroxyl groups. (b) Schematic diagram for the synthesis of N-GQDs. Reprinted with permission from: Ref. [65]. Copyright 2014 Elsevier; Ref. [66]. Copyright 2018 ACS.
4. Direct Chemical Cutting Process
Broad range of carbon or biomass-derived char are been utilized to produce QDs using direct chemical cutting technique. So, comparably to electrochemical process, this technique engages less initial material cost which reduces total operation expenses. Chemical cutting is been classified into two individual types. First one involves single-stage cutting of carbon materials via applying strong acids and the other category is a two-stage process which is comprised of combinational approach of Hummers method and then chemical reduction process to finally produce QDs. Coal and graphene like carbon sources are frequently used in the single-stage process for synthesis. Chemical cutting is been frequently used to produce both CQDs and GQDs. Using graphene along with nitric acid and sulfuric acid concentrated solution, chemical cutting process can be used to develop GQDs [67]. These acids act as suitable cutter for exfoliating GQDs from various precursors. Afterwards, removal of acid is minutely done by calcining the dried mixture to properly separate the QDs. Apart from conventional precursors, nature derived material like chitosan is been examined and proven to be suitable for preparing doped GQDS [64]. Preparing GQDs using walnut shells along with similar concentrated acid solutions (nitric acid and sulfuric acid) is another novel study which can attract authors to study more different waste materials to produce GQDs via using this process [68,69]. Studies have also examined preparation of CQDs. Precursors like coal, gelatin, are been commonly used to prepare the long chain ligands at the quantum dot surface that can inhibit efficient charge transfer. As, the long chains may increase the pathway of electron transfer, reducing the charge kinetics. The ligands possessing multiple groups on the surface can stability or solubility of composites. The sensitivity and selectivity of the QDs can also be regulated by the ligands attached to the surface. But it depends on the pH medium and salt concentration so regulate the storage ability [70]. Overall, this process can considerably attractive owing to its low cost and simplicity. Yet, few drawbacks were also observed such as longer reaction period, usage of hazardous acids for cutting the precursor layers, difficulties faced during acidic solvent after synthesis etc. These problematic factors not only affect the synthesis but also pose harmful environmental threats.
5. Hummers Method
Utilization of Hummers method or modified Hummers method is extensively preferred to synthesize GQDs. The process is generally divided into two segments; first one involves preparation of graphene oxide (GO) and then chemical cutting procedure is used to finally prepare GQDs [71,72,73,74]. For an instance, Shen et al. utilized natural graphite powder to synthesize GO via employing modified Hummers method and afterwards heated the as-prepared GO in the nitric acid solution for 24 h to prepare the GQDs [68]. Similarly, Li et al. prepared nitrogen doped GO via the Hummers method and employed chemical cutting process by heating the prepared GO in a concentrated solution of nitric acid and sulfuric acid to finally synthesize N-GQDs [73]. Maintaining similar procedures, coal is also been used along with Hummers method to prepare GQDs. So, it is clearly observed in the reports that usage of Hummers method seems to be more purposeful when it is used jointly with chemical cutting process.
Compositing QDs with polymer materials are done by few precise processes such as in situ chemical polymerization, doping methods, solution phase mixing method or simple mixing and filtration methods, direct photo-electrodeposition techniques etc. [18,36,37,75,76,77,78,79]. For example, Devadas and Imae, CTAB, APS along with carbon dots to prepare the composite [80]. Firstly, the CTAB was dissolved in aqueous hydrochloric acid solution and stirred properly. Afterwards, the as-prepared carbon dots solution was added and then Sequentially pyrrole monomer added and whole combination was kept stirring for 30 min with maintaining 0–5 deg. Lastly, APS in aqueous HCl solution was added and filtered using a cellulose acetate membrane filter to obtain final composite product. In another study, Arthisree and Madhuri used the solution phase mixing method to combine PAN dissolved in DMF, PANI and GQD dispersed in DMF with different concentration (PAN: PANI: GQD ¼ 9:1:0, 9:0.5:0.5, 8:1:1, 7:1.5:1.5) and stirred for 12 h at room temperature [41]. Afterwards via drop casting technique, the synthesized composite solutions were spread uniformly on to a glass petri dish and dried for about 76 h at room temperature to finally formed the films to apply for electrochemical studies. Apart from these methods, pyrolysis is another facile technique been successfully used to synthesize core-shell structure of C-QDs coated by PANI [81]. However, for this particular composite preparation these methods need to be further explored to accomplish more compact form and structure.
This entry is adapted from the peer-reviewed paper 10.3390/polym14051053
This entry is offline, you can click here to edit this entry!
